Abstract
The U.S. population has nearly one radiographic examination per person per year and concern about cancer risks associated with medical radiation has increased. Radiologic technologists were surveyed to determine whether their personal cumulative exposure to diagnostic x-rays was associated with increased frequencies of chromosome translocations, an established radiation biomarker and possible intermediary suggesting increased cancer risk. Within a large cohort of U. S. radiologic technologists, 150 provided a blood sample for whole chromosome painting and were interviewed about past x-ray examinations. The number and types of examinations reported were converted to a red bone marrow (RBM) dose score with units that approximated 1 mGy. The relationship between dose score and chromosome translocation frequency was assessed using Poisson regression. The estimated mean cumulative RBM radiation dose score was 49 (range 0 – 303). After adjustment for age, translocation frequencies significantly increased with increasing RBM dose score with an estimate of 0.004 translocations per 100 cell equivalents per score unit (95% confidence interval 0.002 to 0.007; P < 0.001). Removing extreme values or adjustment for gender, cigarette smoking, occupational radiation dose, allowing practice x-rays while training, work with radioisotopes, and radiotherapy for benign conditions did not affect the estimate. Cumulative radiation exposure from routine x-ray examinations was associated independently with increased chromosome damage, suggesting the possibility of elevated long-term health risks, including cancer. The slope estimate was consistent with expectation based on cytogenetic experience and atomic bomb survivor data.
Keywords: Radiation exposure, diagnostic x-rays, chromosome translocations, FISH, risk factors
Introduction
Routine medical diagnostic x-ray doses to patients per examination have diminished over time; however, there has been a rapid increase in the use of procedures that confer much higher doses, such as helical and other types of computed tomography (CT) scanning, and interventional procedures (1–4). The US population has nearly one radiographic examination per person per year (2) and new estimates reveal that the average US resident in 2006 was exposed to nearly six times the medical radiation of a person of the same age than in 19891. Much of the increase in medical radiation can be attributed to CT and nuclear medicine procedures. The number of CT scans performed in the US has increased from 18.3 million in 1993 to 62 million in 20061. Further, concern about medical radiation and long-term cancer risks has drawn increased attention (3, 5–6). Excess cancer risks related to diagnostic x-rays were indirectly quantified for 15 countries; the proportions of cancers in the US and the UK attributed to diagnostic radiation were estimated to be 0.9% and 0.6%, respectively (7). Direct estimates of cancer risk associated with diagnostic x-ray examinations are problematic due to low doses, absence of radiographic records, long disease latency, and difficulty in individual recall of specific procedures (2).
Cytogenetic damage is caused by radiation exposure and chromosome aberrations have been associated with increased cancer risk (8). Some studies have found increased chromosome abnormalities immediately after radiation exposure from CT scanning (9) or in patients with unusually high numbers of diagnostic procedures (10). To our knowledge, studies to detect chromosome damage from long-term cumulative low radiation doses (under 0.3 Gray (Gy) or equivalently 300 mGy) from medical radiographic examinations have rarely been attempted because of potential inaccuracies in self-reported x-ray procedures and because the doses were considered to be under the cytogenetic detection limit (about 300 mGy). However, lowering the detection limit may be achieved (11) with careful subject selection, detailed questionnaire information, large sample size (>100 persons), evaluating huge numbers of cells per person (at least 1000 cell equivalents), and increasing the number of chromosomes analyzed. Error in recalling diagnostic procedures might be mitigated by studying radiologic technologists who probably are more knowledgeable than the general public about past x-ray examinations.
Structural chromosome aberrations, specifically translocations enumerated using whole chromosome paints by fluorescence in situ hybridization (FISH) in peripheral blood lymphocytes, have been used extensively as biodosimeters of past radiation exposure (12–21). We recently assessed archived translocation data, from over 10 years ago, detected by FISH whole chromosome painting with respect to reported personal diagnostic x-ray examinations and found a significant positive dose-response relationship (p = 0.01) (22). To determine more definitively whether chromosome damage was associated with self-reports of routine diagnostic x-ray examinations, we expanded our earlier study by nearly doubling the number of subjects from 79 radiologic technologists in the first study to 150 in the current study. We also increased the number of cell equivalents evaluated from 432 to approximately 1024 per person in the current study. Here we report our findings of chromosome translocation frequencies and the association with cumulative lifetime diagnostic red bone marrow (RBM) radiation exposure.
Methods
Study population
In 1982, the National Cancer Institute, in collaboration with the University of Minnesota and the American Registry of Radiologic Technologists, initiated a study of cancer incidence and mortality among 146,022 U.S. radiologic technologists (USRT) who were certified for at least two years between 1926 and 1982. This study has been approved annually by the human subjects review boards of the National Cancer Institute and the University of Minnesota. In brief, during 1984–89, 1995–98, and 2003–05, postal surveys were conducted that included questions related to several health outcomes, work history, cancer risk factors, and history of diagnostic x-ray procedures (for questionnaires, see2; for study details, see 23). To date, 110,418 technologists have responded to one or more surveys that formed the basis for several reports of cancer incidence associated with a history of working as a radiologic technologist (24–26).
Subject selection and recruitment for the USRT biodosimetry study have been described in detail elsewhere (21). Briefly, technologists were selected based on occupational characteristics from among a core group of 3441 cohort members who began working before 1950, were alive, and had a known address in 2003. A pre-screening questionnaire identified and excluded cohort members with a prior cancer diagnosis, a family history of chromosomal instability disorders, or who reported smoking 10 or more cigarettes per day. After these exclusions, 207 eligible technologists were serially recruited and 159 (77%) agreed to participate by providing a venipuncture blood sample and completing a telephone survey about personal history of diagnostic and therapeutic radiologic procedures. In-home blood samples were collected using a nation-wide phlebotomy service and shipped overnight to the cytogenetics laboratory. The final sample size was 150 individuals because two subjects died after blood sampling but before their interview, two samples were unusable due to delays in shipment, and five samples did not grow in cell culture.
FISH Assay for Chromosome Aberrations
Laboratory personnel (DP and JDT) determined the frequency of translocations using FISH whole chromosome painting without knowledge of ionizing radiation exposure of the technologists. Cell cultures were initiated on blood collected in heparinized vacutainer tubes within 24 hours of phlebotomy and processed according to routine cytogenetic methods (27). The slide preparation, staining and cell scoring were performed using standardized chromosome painting protocols (27–28).
Chromosomes 1, 2, and 4 were painted red and 3, 5, and 6 were painted simultaneously in green using probes from Cytocell Technologies, Ltd. (Cambridge, UK). The slides were counterstained with 4′,6-Diamidino-2-phenylindole. This combination of paints detects 56% of all the chromosome exchanges (29–30). Only well-spread metaphase cells that met established criteria (31) were scored. All chromosome aberrations were classified according to the Protocol for Aberration Identification and Nomenclature Terminology (PAINT) (32). Approximately 1,800 metaphase cells were evaluated per subject, and this was equivalent to 1,800 * 0.56 = 1,000 metaphase cells (defined as cell equivalents (CEs)) as if the full genome had been scored. All translocations in cells were enumerated and the frequency of translocations per 100 CEs was used as the dependent variable in the statistical analyses.
Occupational Radiation Exposure
The USRT dosimetry system provides annual RBM doses for each technologist as log-normally distributed probability densities for each year that they worked (33). We summed the mean values of each annual distribution to derive cumulative mean occupational RBM radiation doses for adjustment in the statistical analysis (for further detail, see 21). A summary of the most current occupational dose distribution is shown in Table 1 and reflects some dosimetry improvements recently implemented.
Table 1.
Distribution of covariates among biodosimetry study subjects, mean translocation frequencies, and rate ratios by covariate categories, U.S. Radiologic Technologists Study
| Characteristic | Subjects (n = 150*) | Mean number of translocations per 100 CEs | Translocation Rate Ratios† (95% CI) |
|---|---|---|---|
| Age at blood draw‡ | |||
| 71–74 | 17 (11%) | 0.9 | 0.7 (0.5, 1.0) |
| 75–78 | 59 (39%) | 1.4 | 1.0 (referent) |
| 79–82 | 31 (21%) | 1.3 | 1.0 (0.8, 1.2) |
| 83–86 | 31 (21%) | 1.5 | 1.1 (0.9, 1.4) |
| 87–90 | 12 ( 8%) | 1.9 | 1.5 (1.1, 2.0) |
| Gender | |||
| Female | 104 (69%) | 1.2 | 1.0 (referent) |
| Male | 46 (31%) | 1.7 | 1.3 (1.1, 1.6) |
| Race | |||
| Caucasian | 147 (98%) | 1.4 | N/A |
| African American | 1 (<1%) | N/A | N/A |
| Other | 2 (1%) | N/A | N/A |
| Former cigarette smoking (number of pack years) | |||
| 0 | 87 (58%) | 1.4 | 1.0 (referent) |
| >0 – 20 | 31 (21%) | 1.2 | 1.1 (0.8, 1.5) |
| >20 – 50 | 17 (11%) | 1.5 | 1.2 (0.9, 1.6) |
| >50 | 14 ( 9%) | 1.6 | 1.7 (0.8, 3.7) |
| History of x-ray therapy for benign conditions | |||
| Never | 133 (89%) | 1.4 | 1.0 (referent) |
| Ever | 17 (11%) | 1.6 | 1.2 (0.9, 1.5) |
| In the past, allowed others to take practice x-rays | |||
| Never | 88 (59%) | 1.3 | 1.0 (referent) |
| 1 – 24 times | 35 (23%) | 1.4 | 1.0 (0.8, 1.3) |
| ≥ 25 times | 15 (10%) | 1.8 | 1.3 (1.0, 1.7) |
| Estimated occupational radiation dose to the red bone marrow§ | |||
| ≤10 mGy | 53 (35%) | 1.1 | 1.0 (referent) |
| >10 – 20 mGy | 43 (29%) | 1.3 | 1.1 (0.9, 1.5) |
| >20 – 30 mGy | 27 (18%) | 1.7 | 1.5 (1.2, 2.0) |
| >30 – 40 mGy | 17 (11%) | 1.5 | 1.4 (1.0, 1.8) |
| >40 mGy | 10 ( 7%) | 1.7 | 1.4 (1.0, 2.0) |
| Cumulative personal diagnostic red bone marrow radiation dose score** | |||
| 0 – 20 | 51 (34%) | 1.2 | 1.0 (referent) |
| >20 – 50 | 51 (34%) | 1.3 | 1.1 (0.9, 1.4) |
| >50 – 100 | 28 (17%) | 1.3 | 1.1 (0.9, 1.4) |
| >100 | 20 (13%) | 2.0 | 1.6 (1.2, 2.0) |
Abbreviations: CE-Cell equivalents; in cytogenetic studies of radiation-exposed individuals it is common to express the translocation frequency as per 100 or per 1000 CEs as this allows a comparison with other laboratories that may have painted a different combination of chromosomes. CI-Confidence Interval. N/A-Not applicable.
Does not always sum to 150 or 100% due to small numbers and percentages in the unknown category: Smoking, n=1; worked with radioisotopes, n=2; in the past allowed others to take practice x-rays, n=12.
Poisson regression adjusted for age; unknowns not included.
P-trend = <0.001 from Poisson regression with ordinal age categories treated as a continuous variable
P-trend = 0.006 from age-adjusted Poisson regression with ordinal estimated occupational red bone marrow dose categories as a continuous variable
P-trend = 0.001 from age-adjusted Poisson regression with ordinal estimated personal diagnostic red bone marrow dose score categories treated as a continuous variable. One dose score unit is approximately equivalent to 1 mGy
Personal diagnostic exposure
We used self-reported information about personal diagnostic x-ray procedures to assign weighted RBM dose scores. The doses associated with specific radiographic procedures were assigned midpoint RBM dose values from a comprehensive list of examination types (34). We used the mid-point doses and frequencies to weight the dose scores for the types of radiographic procedures reported by the technologists (see Table 2 and footnotes), multiplied the number of examinations by the corresponding dose and then summed the doses to estimate the total cumulative personal diagnostic x-ray weighted RBM dose score. While one unit of the dose score is approximately 1 mGy, because of uncertainties in recall and dose estimates of the procedures, we prefer the term “cumulative red bone marrow radiation dose score” rather than dose per se. The RBM radiation dose from one chest CT scan would roughly correspond to 10 mGy or 10 units of the cumulative RBM dose score.
Table 2.
Estimated red bone marrow (RBM) radiation doses for personal diagnostic x-ray procedures reported by technologists and the corresponding weighted RBM radiation dose score, U.S. Radiologic Technologists Study
| Examination type reported by technologists* | Examination type included in Preston- Martin et al, 2003† | Red bone marrow (RBM) dose (mGy) per examination in Preston-Martin et al, 2003† | Proportion of examinations in Preston-Martin et al, 2003† by grouped category | Number of examinations reported by radiologic technologists (total = 5287) | Proportion of total examinations reported by radiologic technologists | Weighted RBM dose score assigned to examinations reported by technologists‡ |
|---|---|---|---|---|---|---|
| Cervical spine | 0.1 | 0.55 | ||||
| Facial/nasal bones | 0.1 | 0.04 | ||||
| Face or Neck X-ray | Mandible | 0.1 | 0.02 | 410 | 0.08 | 0.2 |
| Neck soft tissue | 0.1 | 0.02 | ||||
| Sinuses | 0.1 | 0.26 | ||||
| Skull | 0.5 | 0.11 | ||||
| Entire spine | 2.4 | 0.06 | ||||
| Lumbar spine | 1.5 | 0.24 | ||||
| Lumbosacral spine | 1.1 | 0.44 | ||||
| Spine or Back X-ray | Myelogram | 3.7 | 0.05 | 711 | 0.13 | 1.4 |
| Sacrum/coccyx | 1.7 | 0.02 | ||||
| Thoracic | 0.7 | 0.14 | ||||
| Thoracic/cervical spine | 0.4 | 0.01 | ||||
| Thoraco-lumbar spine | 2.4 | 0.03 | ||||
| Abdomen | 0.4 | 0.27 | ||||
| Barium enema | 6.3 | 0.16 | ||||
| Cholangiogram | 2.3 | 0.02 | ||||
| Cholecystogram | 1.6 | 0.03 | ||||
| Colon | 1.3 | 0 | ||||
| GI or Abdomen X-ray | Endoscopic Retrograde Cholangiopancreatography | 4.2 | 0.01 | 641 | 0.12 | 3.8 |
| Esophagram | 4.1 | 0.01 | ||||
| GI tract | 0.7 | 0.01 | ||||
| Lower GI Series | 6.3 | 0.2 | ||||
| Pancreas | 1.0 | 0 | ||||
| Small bowel series | 3.5 | 0 | ||||
| Small bowel | 3.5 | 0 | ||||
| Upper GI series | 3.6 | 0.23 | ||||
| Upper GI + esophagram | 7.7 | 0.02 | ||||
| Upper GI + small bowel | 7.1 | 0.03 | ||||
| Cystogram | 1.7 | 0.01 | ||||
| Kidneys/ureters/bladder | 0.5 | 0.32 | ||||
| Urinary System X-ray | Pyelogram, intravenous | 2.1 | 0.59 | 138 | 0.03 | 1.6 |
| Pyelogram, retrograde | 2.2 | 0.07 | ||||
| Urethrogram | 2.6 | 0.01 | ||||
| Hip | 0.4 | 0.42 | ||||
| Bony Pelvis or Hip X-ray | Pelvis | 0.4 | 0.43 | 385 | 0.07 | 0.4 |
| Pelvis/hips | 0.6 | 0.15 | ||||
| Chest | 0.05 | 0.97 | ||||
| Chest X-ray | Ribs | 0.4 | 0.02 | 2463 | 0.47 | 0.1 |
| Sternum | 0.6 | 0.01 | ||||
| Chest Fluoroscopy | Chest, Fluoroscopic | 1.4 | NA§ | 206 | 0.04 | 1.4 |
| Cerebral arteriogram | Cerebral arteriogram | 1.0 | NA | 23 | 0.00 | 1.0 |
| Carotid arteriogram | Cerebral arteriogram | 1.0 | NA | 15 | 0.00 | 1.0 |
| Pul. Arteriogram | Pulmonary arteriogram | 1.4 | NA | 4 | 0.0 | 1.4 |
| Renal Arteriogram | Renal arteriogram | 2.1 | NA | 5 | 0.00 | 2.1 |
| Coronary Angiogram | Coronary angiogram | 12.7 | NA | 91 | 0.02 | 12.7 |
| Head or neck CT | Head/neck | 3.0 | NA | 65 | 0.01 | 3.0 |
| Chest CT | Chest | 9.6 | NA | 43 | 0.01 | 9.6 |
| Abdomen CT | Abdomen | 14.6 | NA | 36 | 0.01 | 14.6 |
| Pelvis CT | Pelvis | 9.1 | NA | 6 | 0.00 | 9.1 |
| Upper Back CT | Thoracic spine | 2.6 | NA | 6 | 0.00 | 2.6 |
| Lower Back CT | Lumbar spine | 5.6 | 0.56 | 31 | 0.01 | 4.8 |
| Lumbosacral spine | 3.9 | 0.44 | ||||
| Whole Body CT | Whole body | 3.5 | NA | 8 | 0.00 | 3.5 |
Ascertained from telephone interview at the time of blood collection
Reported by Preston-Martin and Pogoda (2003)
Determined by weighting each Preston-Martin and Pogoda (2003) exam dose by the frequency with which the exam type occurred in their study population and is an approximation of 1 mGy, because of the uncertainties in recall of various procedures we prefer using the term “diagnostic red bone marrow radiation dose score” rather than standard dose units.
Not applicable
To assess the reproducibility of recall of personal diagnostic procedures by cohort members, we compared self-reports from 354 radiologic technologists who completed the same questionnaire twice during a four year period. The distributions by age, gender, race, and selected work history characteristics were similar for these technologists when compared with the full study cohort. We evaluated the number of reported procedures and the decade they first occurred for the following common or high dose examinations: upper gastrointestinal series, angiography, CT scans, mammography (among women age 50 or older), and chest x-rays.
Personal therapeutic radiation exposure
We included an indicator variable in the statistical model for having therapeutic irradiation (ever/never). Information on personal history of therapeutic irradiation to the head and neck, pelvis, extremities and chest, for benign conditions, was available from the baseline questionnaire. Because the majority of adult bone marrow is located in the pelvis, torso, and head (35), we categorized those who had therapeutic radiation to the extremities as “never”. Those categorized as “ever” reported treatment for the following benign conditions: acne (n=6), shoulder pain (n=3), skin cancer (n=2), boils (n=2), and other varied reasons (n=4).
Statistical Analysis
We used the AMFIT module of EPICURE (Hirosoft, Seattle, WA) to construct linear Poisson regression models for associations between cumulative personal diagnostic x-ray weighted RBM dose score and translocation frequency. The models were of the following general form:
where λ is the expected number of translocations per cell, a represents covariates affecting translocation frequency and d is the personal diagnostic x-ray RBM dose, λ0(a) is the covariate specific background number of translocations per cell, and β is the increase in translocations per cell per unit dose score. A Pearson scale factor was added to the models to account for over-dispersion of the data.
Selected covariates were included in a multivariate model in order to minimize confounding. Potential confounders were covariates whose inclusion in the model changed the association between weighted RBM dose score and translocation frequency, i.e. parameter β, by 10% or more. The final model included the covariate age at blood draw, but not gender, race, cigarette smoking, prior therapeutic radiation, a past history of allowing others to take practice x-rays, or occupational radiation dose. For ordinal variables, tests for trend were performed in univariate linear Poisson regression models using categories as continuous variables.
To determine if the observed dose-response relationship was influenced by outliers, we excluded seven individuals with the highest cumulative medical diagnostic x-ray RBM doses and/or the highest translocation frequencies and evaluated the effect on the slope estimate. We individually examined the work histories for those persons with extreme translocation values and found no unusual characteristics, although the technologist with the highest number of translocations reported working almost exclusively with radioisotopes. We found very little effect of the exclusions so that in the final analyses we included all subjects.
Results
Descriptive features of the study population are shown in Table 1. Technologists ranged in age from 71 to 90 years (median 78), were predominately female (69%), Caucasian (98%), and non-smokers (58%). All began working in the 1940s, and worked an average of 22 (range 1 to 49) years (data not shown). Eleven percent reported having therapeutic radiation for benign conditions to the pelvis, torso or head; adult body sites with the most RBM.
Mean translocations by categories of covariates and age-adjusted translocation rate ratios comparing categories of these covariates are also presented in Table 1. The average number of translocations increased with age (p-trend < 0.001), male gender (vs. female, RR=1.7; 95% confidence interval (CI) 1.1, 1.6), a past history of allowing others to take practice x-rays during technologist training (25 times or more vs. never, RR=1.3, 95% CI 1.0, 1.7), occupational RBM dose (p-trend = 0.006), and personal diagnostic RBM radiation dose score (p-trend = 0.001). Translocation frequencies were not statistically significantly different by pack-years of former cigarette smoking, working with radioisotopes (data not shown), or a history of x-ray therapy for benign conditions.
Study technologists had an average of 1.4 translocations/100 CEs (median 1.2, range 0–4.5), a mean occupational RBM dose of 17 mGy (median 14, range 0.6–56), and a mean cumulative personal diagnostic RBM radiation dose score of 49 (median 33, range 0–303). Personal diagnostic x-ray RBM dose score was uncorrelated with occupational RBM dose (r2=0.02). The distribution of the collective (all subjects) diagnostic RBM radiation dose score was 9.4% before 1950 (collective score = 681), 18.8% between 1950 and 1969 (collective score = 1365), and 71.9% after 1969 (collective score = 5228). Translocation frequencies plotted against diagnostic RBM radiation dose score are shown in Figure 1. In the unadjusted analysis, a one unit increase in personal radiographic radiation dose score was associated with an increase of 0.004 excess translocations/100 CEs (95% confidence interval, 0.002–0.007; p = 0.001). In the analysis adjusted for age at blood collection, the estimate remained unchanged at 0.004 excess translocations/100 CEs (p < 0.001). Subsequently adding radiation exposure from therapeutic procedures to the model or dropping extreme values did not alter the point estimate.
Figure 1.
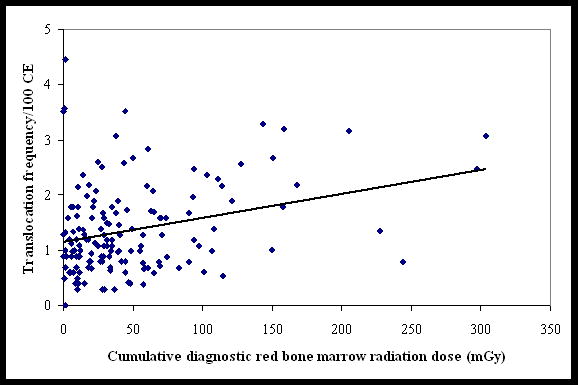
Translocation frequency versus the cumulative diagnostic red bone marrow radiation dose score among 150 U.S. radiologic technologists. (The trend line is from univariate Poisson regression analysis (0.004 translocations/100 CEs/RBM dose score, p < 0.001))
Consistency in reporting the numbers of personal diagnostic procedures ranged from agreement of 51% for chest x-rays (within two procedures) to 100% for angiography. If the criteria for chest x-ray agreement was relaxed (within five), the agreement reached 92%. Agreement for the number of mammograms was 68%. Agreement for decade in which the reported procedure first occurred ranged from 79% for upper gastrointestinal series to 86% for angiography. Agreement percentages for decade of first chest x-ray and first mammogram were 84% and 81%, respectively.
Discussion
We observed a statistically significant increase in translocation frequency (p = 0.001) with increasing cumulative diagnostic RBM radiation dose score after adjusting for age. The estimate for the slope was unchanged and independent of radiation dose from occupational exposures, as evidenced by multivariate analysis that included several potential confounding variables. We are among the first to show that accumulated radiation doses from a lifetime of routine x-ray examinations was statistically significantly associated with increased cytogenetic damage in the form of chromosome translocations. Extreme values do not explain our finding because the association remained robust after excluding outliers.
Our ability to detect a low-dose relationship, despite the older ages of the radiologic technologists, their relatively low cumulative personal diagnostic RBM radiation dose scores, and the substantial inter-individual variability in translocation frequencies, was enhanced by study features designed to overcome these limitations (11, 36). The features were the very large sample size, the scoring of 1828 cells on average (1024 cell equivalents) per person (274,234 cells in the whole study), and the ability to select participants who were homogeneous for age. Additionally, we tried to reduce the effect of cigarette smoking on translocation frequencies by restricting the selected sample to non- or ex-smokers. While previous studies examining the influence of cigarette smoking on translocation frequency have not been consistent (13, 16, 19, 27, 37–39), a recent pooled study demonstrated a significant increased frequency of chromosome translocations associated with ever smoking (40).
An alternative explanation for why we detected a low-dose relationship may be that we did not lower the limits of detection, but rather the radiation doses were in fact higher because the radiologic technologists under-reported x-ray examinations (41–42) or repeated x-rays were performed when images were of low quality. Our analysis of the reproducibility of self-reporting among 354 technologists who completed the same questionnaire twice within a four year period showed an overall 80% agreement, suggesting radiologic technologists are consistent in their recall of the number of past diagnostic procedures (78% agreement) and the time period they first occurred (83% agreement).
The cytogenetic damage we detected appeared to be associated with more recent routine x-ray examinations because 72% of the collective cumulative medical RBM radiation dose scores occurred after 1969. This is of concern because of the large increase in medical radiation exposure since 19891 and because increased frequencies of chromosome aberrations have been associated with elevated cancer risk (reviewed in 8). While the link between radiation exposure from personal diagnostic procedures, chromosome aberrations, and cancer risk is indirect, ionizing radiation is a well known carcinogen.
In studies of high energy gamma rays, the expected frequency of excess translocations per 100 CEs per 1 mGy is 0.0015 (43). The distribution of applicable photon energies in the present study were x-rays of approximately 100 keV or less. For dicentrics, the unstable counterpart of translocations, the linear term for x-rays of 50–100 keV is about two to three times higher than that for high energy gamma-rays (44). So, for the relevant energies here, an estimated frequency of 0.005 excess translocations/100 CE/mGy is reasonable. If one exposure score unit approximates 1 mGy, the observed association of 0.004 excess translocations/100 CEs/unit diagnostic RBM radiation dose score (95% CI, 0.002–0.007) was consistent with this estimate and generally similar to the occupational RBM estimate of 0.009 excess translocations/100 CE/mGy (95% CI -0.001–0.02, p = 0.07) in the same group of technologists (21). Among the 79 technologists for whom FISH analysis was done over a decade earlier, we estimated 0.007 translocations per 100 CEs per score unit (95% CI, 0.002 to 0.013; P=0.01, (22)). Lending further support to our findings are estimates from Hiroshima and Nagasaki atomic bomb survivors in which the proportion of cells with at least one stable aberration, a similar measure considering the generally low frequencies of translocations, increased by about 0.007/100 CE/mGy and 0.004/100 CE/mGy RBM absorbed dose, respectively (17). Acknowledging the dosimetric uncertainties in both studies, similar estimates might be expected because the effect of exposure to higher energy gamma rays in the atomic bomb survivors (45) could be counterbalanced by the protracted radiation exposure in the radiologic technologists.
Recall error of past diagnostic x-ray procedures has been a legitimate obstacle for conducting large population-based health outcome studies relying on self-report (2). However, because the technologists did not know their translocation frequencies, any recall error would be non-differential with respect to the outcome measure and would probably attenuate relationships observed, not create them. We lacked information on environmental toxins that could be related to increased chromosome translocations, however these unmeasured variables would also need to be related to medical x-ray examinations to confound the relationship we observed. Given the absence of confounding for several candidate variables we measured and analyzed, the masking of a true association with an unmeasured co-variate is unlikely.
This is one of the first studies to report a significant association between chromosome translocations and estimated cumulative diagnostic RBM radiation exposure. We found that radiation from routine x-ray examinations among radiologic technologists was associated with increased chromosome damage, which has been related to elevated cancer risk. Further, the magnitude of the relationship was consistent with expectation based on knowledge of radiation quality and cytogenetic experience. In an earlier study, we found similar results based on 79 radiologic technologists from the same cohort for whom432 whole genome equivalents per person were analyzed (22). The present work, which was done in a different laboratory, improves on the first study by increasing the sample size and the number of cells examined per person. While disease diagnosis and patient treatment have been markedly improved by medical uses of radiation, the dose to the individual from diagnostic tests should be monitored and reduced when possible. Our data indicate the need for careful evaluation before recommending diagnostic radiologic examinations, especially in light of the recent and substantial proliferation in the US of high-dose examinations, including CT and nuclear medicine procedures. For example, incidental findings of uncertain clinical significance on CTs are followed by additional scans that may not result in patient benefit. Dose reduction can be achieved without lowering diagnostic accuracy1, making judicious choices by understanding effective doses from each of the various procedures (4), oversight of cumulative patient doses, and the avoidance of repeats of poor initial quality diagnostic x-ray examinations.
Acknowledgments
We are grateful to the radiologic technologists who participated in the USRT Study; Jerry Reid of the American Registry of Radiologic Technologists for continued support of this study; Allison Iwan of the University of Minnesota for data collection and study coordination; Laura Bowen of Information Management Services, Inc (Silver Spring, MD) for data management and computing; Christopher McClure of Research Triangle International, Inc. (Research Triangle Park, NC) for overseeing in-home blood collection and biospecimen transport. We appreciate the technical assistance of Homa Chuku, Sujatha Gajapathy, Alex Husseini, Michael Mitchell, and Lindsey Schulz in scoring the slides for translocation frequencies.
Funding information: This project was funded in part by the Intramural Research Program of the Division of Cancer Epidemiology and Genetics, the National Cancer Institute, National Institutes of Health, Department of Health and Human Services and by an interagency agreement with the National Institute for Occupational Safety and Health contract Y1-CP-9012. The findings and conclusions in this report are those of the author(s) and do not necessarily represent the views of the National Institute for Occupational Safety and Health.
Abbreviations
- RBM
red bone marrow
- FISH
fluorescence in situ hybridization
- CE
cell equivalents
- CI
confidence interval
- USRT
United States radiologic technologists
- Gy
Gray
- Sv
Sievert
Footnotes
The authors declare they have no competing financial interests.
References
- 1.Mettler FA, Jr, Briggs JE, Carchman R, Altobelli KK, Hart BL, Kelsey CA. Use of radiology in U.S. general short-term hospitals: 1980–1990. Radiol. 1993;189:377–80. doi: 10.1148/radiology.189.2.8210363. [DOI] [PubMed] [Google Scholar]
- 2.Ron E. Cancer risks from medical radiation. Health Phys. 2003;85:47–59. doi: 10.1097/00004032-200307000-00011. [DOI] [PubMed] [Google Scholar]
- 3.Brenner DJ, Hall EJ. Computed tomography—an increasing source of radiation exposure. N Engl J Med. 2007;57:2277–84. doi: 10.1056/NEJMra072149. [DOI] [PubMed] [Google Scholar]
- 4.Mettler FA, Huda W, Yoshizumi TT, Mahesh M. Effective doses in radiology and diagnostic nuclear medicine: a catalog. Radiol. 2008;248:254–263. doi: 10.1148/radiol.2481071451. [DOI] [PubMed] [Google Scholar]
- 5.Hampton T. Researchers examine long-term risks of exposure to medical radiation. In “Medical news and perspectives”. J Am Med Assoc. 2006;296:638–40. doi: 10.1001/jama.296.6.638. [DOI] [PubMed] [Google Scholar]
- 6.Einstein AJ, Henzlova MJ, Rajagopalan S. Estimating risk of cancer associated with radiation exposure from 62-slice computed tomography coronary angiography. J Am Med Assoc. 2007;298:317–23. doi: 10.1001/jama.298.3.317. [DOI] [PubMed] [Google Scholar]
- 7.Berrington de González A, Darby S. Risk of cancer from diagnostic X-rays: estimates for the UK and 14 other countries. Lancet. 2006;9406:345–51. doi: 10.1016/S0140-6736(04)15433-0. [DOI] [PubMed] [Google Scholar]
- 8.Norppa H, Bonassi S, Hansteen IL, et al. Chromosomal aberrations and SCEs as biomarkers of cancer risk. Mutat Res. 2006;600:37–45. doi: 10.1016/j.mrfmmm.2006.05.030. [DOI] [PubMed] [Google Scholar]
- 9.M’kacher R, Violot D, Aubert B, et al. Premature chromosome condensation associated with fluorescence in situ hybridisation detects cytogenetic abnormalities after a CT scan: evaluation of the low-dose effect. Radiat Prot Dosim. 2006;103:35–40. doi: 10.1093/oxfordjournals.rpd.a006112. [DOI] [PubMed] [Google Scholar]
- 10.Weber J, Scheid W, Traut H. Biological dosimetry after extensive diagnostic x-ray exposure. Health Phys. 1995;68:266–69. doi: 10.1097/00004032-199502000-00012. [DOI] [PubMed] [Google Scholar]
- 11.Tucker JD, Moore DH., II The importance of age and smoking in evaluating adverse cytogenetic effects of exposure to environmental agents. Environ Health Perspect. 1995;104:489–92. doi: 10.1289/ehp.96104s3489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lucas JN, Awa A, Straume T, et al. Rapid translocation frequency analysis in humans decades after exposure to ionizing radiation. Int J Radiat Biol. 1992;62:53–63. doi: 10.1080/09553009214551821. [DOI] [PubMed] [Google Scholar]
- 13.Tucker JD, Tawn EJ, Holdsworth D, et al. Biological dosimetry of radiation workers at the Sellafield nuclear facility. Radiat Res. 1997;148:216–26. [PubMed] [Google Scholar]
- 14.Lindholm C, Tekkel M, Veidebaum T, Ilus T, Salomaa S. Persistence of translocations after accidental exposure to ionizing radiation. Int J Radiat Biol. 1998;74:565–71. doi: 10.1080/095530098141140. [DOI] [PubMed] [Google Scholar]
- 15.Moore DH, II, Tucker JD. Biological dosimetry of Chernobyl clean-up workers: Inclusion of age and smoking data provide improved radiation dose estimates. Radiat Res. 1999;152:655–64. [PubMed] [Google Scholar]
- 16.Burak LE, Kodama Y, Nakano M, et al. FISH examination of lymphocytes from Mayak workers for assessment of translocation induction rate under chronic radiation exposures. Int J Radiat Biol. 2001;77:901–8. doi: 10.1080/09553000110063386. [DOI] [PubMed] [Google Scholar]
- 17.Kodama Y, Pawel D, Nakamura N, et al. Stable chromosome aberrations in atomic bomb survivors: results from 25 years of investigation. Radiat Res. 2001;156:337–46. doi: 10.1667/0033-7587(2001)156[0337:scaiab]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 18.Wang JX, Zhang LA, Li BX, Zhao YC, Wang ZQ, Zhang JY, et al. Cancer incidence and risk estimation among medical x-ray workers in China, 1950–1995. Health Phys. 2002;82:455–66. doi: 10.1097/00004032-200204000-00004. [DOI] [PubMed] [Google Scholar]
- 19.Tawn EJ, Whitehouse CA, Tarone RE. FISH chromosome aberration analysis on retired radiation workers from the Sellafield nuclear facility. Radiat Res. 2004;162:249–56. doi: 10.1667/rr3214. [DOI] [PubMed] [Google Scholar]
- 20.Montoro A, Rodriguez P, Almonacid M, Villaescusa JI, Verdu G, Caballin MR, et al. Biological dosimetry in a group of radiologists by the analysis of dicentrics and translocations. Radiat Res. 2005;164:612–617. doi: 10.1667/rr3444.1. [DOI] [PubMed] [Google Scholar]
- 21.Bhatti P, Preston DL, Doody MM, et al. Retrospective biodosimetry among United States radiologic technologists. Radiat Res. 2007;167:727–34. doi: 10.1667/RR0894.1. [DOI] [PubMed] [Google Scholar]
- 22.Bhatti P, Doody MM, Preston DL, et al. Increased frequency of chromosome translocations associated with diagnostic x-ray examinations. Radiat Res. 2008;170:149–55. doi: 10.1667/RR1422.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sigurdson AJ, Doody MM, Rao RS, et al. Cancer incidence in the US radiologic technologists health study, 1983–1998. Cancer. 2003;97:3080–89. doi: 10.1002/cncr.11444. [DOI] [PubMed] [Google Scholar]
- 24.Freedman DM, Sigurdson AJ, Rao RS, et al. Risk of melanoma among radiologic technologists in the United States. Int J Cancer. 2003;103:556–62. doi: 10.1002/ijc.10854. [DOI] [PubMed] [Google Scholar]
- 25.Linet MS, Freedman DM, Mohan AK, Doody MM, Ron E, Mabuchi K, Alexander BH, Sigurdson AJ, Matanoski GM, Hauptmann M. Incidence of hematopoietic malignancies in U.S. radiologic technologists. Occup Environ Med. 2005;62:861–7. doi: 10.1136/oem.2005.020826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Doody MM, Freedman DM, Alexander BH, et al. Breast cancer incidence in U.S. radiologic technologists. Cancer. 2006;106:2707–15. doi: 10.1002/cncr.21876. [DOI] [PubMed] [Google Scholar]
- 27.Ramsey MJ, Moore DH, Briner JF, et al. The effects of age and lifestyle factors on the accumulation of cytogenetic damage as measured by chromosome painting. Mutat Res. 1995;338:95–106. doi: 10.1016/0921-8734(95)00015-x. [DOI] [PubMed] [Google Scholar]
- 28.Matsumoto K, Ramsey MJ, Nelson DO, Tucker JD. Persistence of radiation-induced translocations in human peripheral blood determined by chromosome painting. Radiat Res. 1998;149:602–13. [PubMed] [Google Scholar]
- 29.Morton N. Parameters of the human genome. Proc Natl Acad Sci. 1991;88:7474–6. doi: 10.1073/pnas.88.17.7474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tucker JD, Breneman JW, Briner JF, Eveleth GG, Langlois RG, Moore DH., II Persistence of radiation-induced translocations in rat peripheral blood determined by chromosome painting. Environ Mol Mutagen. 1997;30:264–72. doi: 10.1002/(sici)1098-2280(1997)30:3<264::aid-em4>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 31.Tucker JD, Cofield J, Matsumoto K, Ramsey MJ, Freeman DC. Persistence of chromosome aberrations following acute radiation: I. PAINT translocations, dicentrics, rings, fragments and insertions (invited paper) Environ Mol Mutagen. 2005;45:229–48. doi: 10.1002/em.20090. [DOI] [PubMed] [Google Scholar]
- 32.Tucker JD, Morgan WF, Awa AA, et al. A proposed system for scoring structural aberrations detected by chromosome painting. Cytogenet Cell Genet. 1995;65:211–21. doi: 10.1159/000133916. [DOI] [PubMed] [Google Scholar]
- 33.Simon SL, Weinstock RM, Doody MM, et al. Estimating historical radiation doses to a cohort of U.S. radiologic technologists. Radiat Res. 2006;166:174–92. doi: 10.1667/RR3433.1. [DOI] [PubMed] [Google Scholar]
- 34.Preston-Martin S, Pogoda JM. Estimation of radiographic doses in a case-control study of acute myelogenous leukemia. Health Phys. 2003;84:245–59. doi: 10.1097/00004032-200302000-00013. [DOI] [PubMed] [Google Scholar]
- 35.Cristy M. Active bone marrow distribution as a function of age in humans. Phys Med Biol. 1981;26:389–400. doi: 10.1088/0031-9155/26/3/003. [DOI] [PubMed] [Google Scholar]
- 36.Brenner DJ. Is the linear-no-threshold hypothesis appropriate for use in radiation protection? Favouring the proposition. Radiat Prot Dosim. 2001;97:279–82. doi: 10.1093/oxfordjournals.rpd.a006675. [DOI] [PubMed] [Google Scholar]
- 37.van Diemen PC, Maasdam D, Vermeulen S, Darroudi F, Natarajan AT. Influence of smoking habits on the frequencies of structural and numerical chromosomal aberrations in human peripheral blood lymphocytes using the fluorescence in situ hybridization (FISH) technique. Mutagenesis. 1995;10:487–95. doi: 10.1093/mutage/10.6.487. [DOI] [PubMed] [Google Scholar]
- 38.Pressl S, Edwards A, Stephan G. The influence of age, sex and smoking habits on the background level of FISH-detected translocations. Mutat Res. 1999;442:89–95. doi: 10.1016/s1383-5718(99)00067-4. [DOI] [PubMed] [Google Scholar]
- 39.Bothwell AM, Whitehouse C, Tawn EJ. The application of FISH for chromosome analysis in relation to cellular exposure. Radiat Prot Dosim. 2000;88:7–14. [Google Scholar]
- 40.Sigurdson AJ, Ha M, Bhatti P, et al. International study of chromosome translocation frequencies among radiation unexposed populations. Mutat Res. 2008;652:112–121. doi: 10.1016/j.mrgentox.2008.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pogoda JM, Preston-Martin S. Radiation exposure from diagnostic imaging: agreement between self-report and medical records. Health Phys. 2002;83:567–70. doi: 10.1097/00004032-200212000-00019. [DOI] [PubMed] [Google Scholar]
- 42.Berrington de González A, Ekbom A, Glass AG, et al. Comparison of documented and recalled histories of exposure to diagnostic x-rays in case-control studies of thyroid cancer. Am J Epidemiol. 2003;157:652–63. doi: 10.1093/aje/kwg026. [DOI] [PubMed] [Google Scholar]
- 43.Edwards AA, Lindholm C, Darroudi F, et al. Review of translocations detected by FISH for retrospective biological dosimetry applications. Radiat Prot Dosim. 2005;113:396–402. doi: 10.1093/rpd/nch452. [DOI] [PubMed] [Google Scholar]
- 44.Guerrero-Carbajal C, Edwards AA, Lloyd DC. Induction of chromosome aberration in human lymphocytes and its dependence on x-ray energy. Radiat Prot Dosim. 2003;106:131–35. doi: 10.1093/oxfordjournals.rpd.a006342. [DOI] [PubMed] [Google Scholar]
- 45.Preston DL, Pierce DA, Shimizu Y, et al. Effect of recent changes in atomic bomb survivor dosimetry on cancer mortality risk estimates. Radiat Res. 2004;162:377–89. doi: 10.1667/rr3232. [DOI] [PubMed] [Google Scholar]


