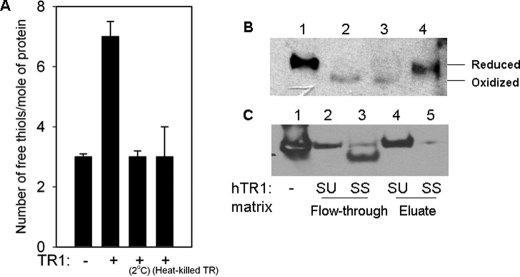
FIGURE 6.
Direct interaction of rTat with TR1 in vitro. A, 2 μm rTat was mixed with 100 nm rat liver TR1 (Sigma) and 0.4 mm NADPH as described under Materials and Methods.” Free thiols were determined using Ellman's reagent (5,5′-dithiobis(2-nitrobenzoic acid). Data shown are the mean of triplicate experiments ±S.D. B, redox Western blot analysis of the reduction of rTat by TR1. The above reaction mixture was reacted with AMS followed by Western blot analysis with anti-Tat monoclonal antibodies. Lanes 1–4 represent DTT-reduced rTat, diamide (N,N,N′,N′-tetramethylazodicarboxamide)-oxidized rTat, native rTat, and TR1-reduced rTat, respectively. Although the protein load was identical in all the lanes, we consistently observed variations in the immunoreactivity of oxidized rTat. C, mechanism-based affinity chromatography showing the binding of rTat to hTR1SU and hTR1SS mutants. Protein in the flow-through and DTT eluate fractions were concentrated and analyzed by Western blot for the presence of Tat. Lanes 1–5 represent rTat positive control, flow-through from SU column, flow-through from SS column, eluate from SU column, and eluate from SS column, respectively.