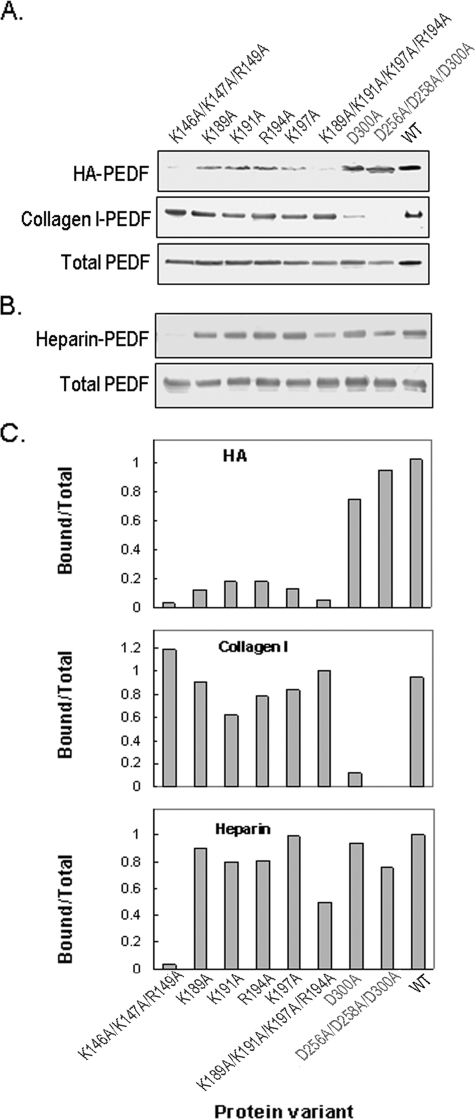
FIGURE 5.
Binding of genetically modified PEDF proteins to HA. BHK cells were transfected with mutated PEDF cDNA expression plasmids. A, culture media (100 μl) of stably transfected cells was concentrated and used in PEDF binding assays with HA (30 μg) and collagen I (2 μg) in 500 μl and 200 μl of phosphate-buffered saline, pH 7.5, respectively. HA-PEDF was isolated by CPC precipitation, and collagen I-PEDF by size exclusion ultrafiltration using centricon-100. PEDF was detected by immunoblotting versus anti-PEDF. HA-PEDF corresponds to PEDF bound to HA; Collagen I-PEDF to PEDF bound to collagen I; and Total PEDF to PEDF in media (20 μl). B, proteins in culture media of stably transfected cells were concentrated to ∼350 μg/ml protein, exchanged to buffer H (0.5 ml) and loaded onto heparin-affinity beads column (0.5 ml bed-volume). The flow-through was reloaded, and the unbound material was washed with 20 column-volumes of buffer H. Bound proteins were eluted with 2 column volumes of 1 m NaCl in buffer H, concentrated by ultrafiltration using centricon-30 devices, and resolved by SDS-PAGE. PEDF was detected by immunoblotting versus anti-PEDF. Heparin-PEDF corresponds to PEDF bound to heparin, and Total PEDF to PEDF in load (6 μg of protein per lane). C, quantification of PEDF bound to HA, collagen I, and heparin. PEDF-immunoreactive bands, as from above, were scanned using UNSCAN-IT software. Values from bound were divided by values from Total protein, plotted as a function of the genetically modified PEDF variant using EXCEL, Microsoft, and are shown.