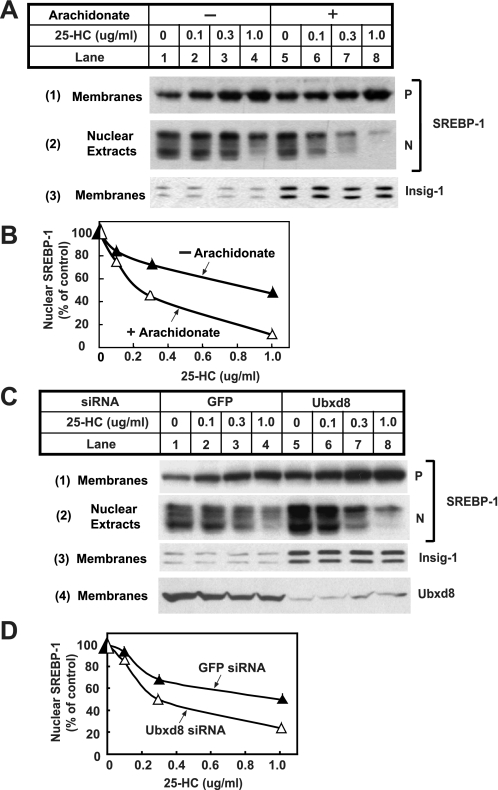
FIGURE 9.
Effects of arachidonate on proteolytic activation of SREBP-1. A, HEK-293S/pInsig1 cells were seeded at 4.0 × 105/60-mm dish on day 0. On day 2, cells were switched to medium B supplemented with 10% delipidated FCS, 50 μm compactin, and 50 μm mevalonate. On day 3, 2 μm LXR agonist T091317 was added into the medium in the absence or presence of 100 μm arachidonate. Following incubation for 6 h, the cells received additions of the indicated amount of 25-hydroxcholesterol (25-HC) and were further incubated for 1 h. Cells were then harvested and fractionated. Aliquots of membrane and nuclear extracts were subjected to SDS-PAGE followed by immunoblot analysis with anti-SREBP1 and anti-T7 (against Insig-1). P and N denote precursor and nuclear forms of SREBP-1, respectively. B, densitometric quantification of the result in A. The intensity of the cleaved nuclear form of SREBP-1 in the absence of 25-hydroxcholesterol was arbitrarily set at 100%. C, HEK-293S/pInsig1 cells were seeded and transfected with the indicated siRNA as described in Fig. 4B. On day 3, cells were switched to medium B containing 10% delipidated FCS with 50 μm compactin and 50 μm mevalonate. On day 4, 2 μm LXR agonist T091317 was added into the medium. Following incubation for 6 h, the cells received additions of the indicated amount of 25-hydroxycholesterol and were further incubated for 1 h. Cells were then harvested and analyzed as described in A. D, densitometric quantification of the result in C as described in B. GFP, green fluorescent protein.