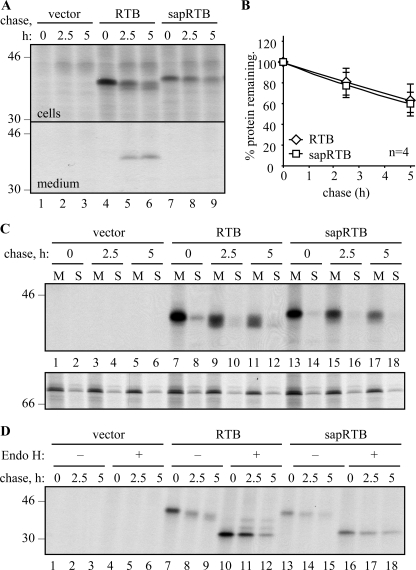
FIGURE 7.
Ricin B chain disappears from the membrane fraction. A, protoplasts were transfected with vector alone (vector), or with plasmids encoding RTB carrying a cleavable phaseolin signal peptide (RTB), or with the saporin signal peptide rendered uncleavable by mutation (sapRTB). Protoplasts were subjected to pulse-chase, and RTB IPs from separated cell homogenates and medium were analyzed by SDS-PAGE and fluorography. B, quantification of RTB was carried out as in Fig. 3. The average of four independent experiments is shown. Error bars indicate standard deviation. C, protoplasts were transfected as in A and subjected to pulse-chase before being homogenized in the absence of detergent and fractionated to yield microsomal membranes (M) and soluble fractions (S). Proteins were sequentially immunoprecipitated using anti-RTB and anti-BiP antisera and analyzed by SDS-PAGE and fluorography. D, protoplasts were transfected as in A and subjected to pulse-chase analysis as before. RTB immunoprecipitates were treated for 16 h in the presence or absence of Endo H before analysis by reducing SDS-PAGE and fluorography. The single and double asterisks indicate the position of Endo H-resistant singly and doubly glycosylated RTB, respectively. In all gel panels, numbers down the margin indicate molecular mass markers in kilodaltons.