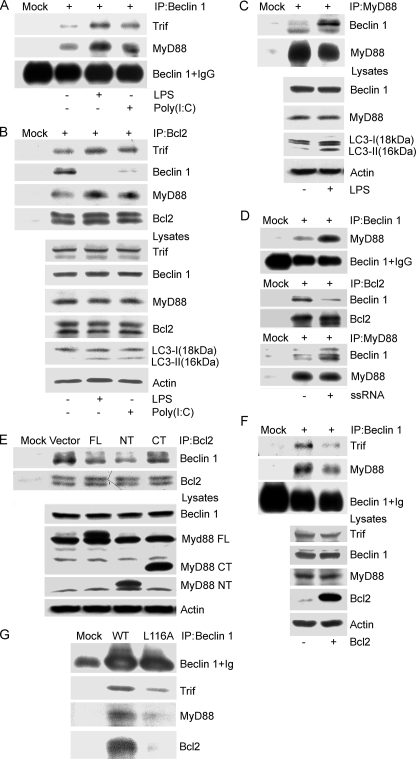
FIGURE 4.
MyD88 and Trif modulate the interaction between Bcl2 and Beclin 1. A, LPS or poly(I·C) stimulation facilitates the association of Beclin 1 with MyD88 and Trif. Lysates from RAW 264.7 cells treated with LPS (100 ng/ml) or poly(I·C) (20 μg/ml) for 4 h were immunoprecipitated (IP) with anti-Beclin 1 antibodies. The resulting immune complexes were analyzed with the indicated antibodies. B, TLR4 and TLR3 activated by LPS and poly(I·C), respectively, promote the association of MyD88 and Trif with Bcl2, and suppress the interaction between Bcl2 and Beclin 1. The cellular lysates from the above preparation and treatment were also immunoprecipitated with a Bcl-2 antibody. The resulting immune complexes and corresponding lysates were analyzed using the indicated antibodies. The induction of autophagy was monitored by immunoblotting with anti-LC3 antibodies. C, LPS stimulation promotes MyD88 association with Beclin 1. RAW 267.4 cells were exposed to LPS (100 ng/ml) for 4 h and the cellular lysates were immunoprecipitated with anti-MyD88 antibody. They along with their corresponding lysate were analyzed by immunoblotting using the indicated antibodies. Autophagy was monitored by LC3 immunoblotting. D, ssRNA40 stimulation promotes MyD88 association with Beclin 1 and decreases Bcl-2 binding to Beclin 1. RAW 267.4 cells were treated with ssRNA40 (10 μg/ml) for 4 h and the cellular lysates were immunoprecipitated with anti-Beclin 1, Bcl-2, or a MyD88 antibody. The indicated proteins were immunoblotted. E, overexpression of FLAG-MyD88 and FLAG-MyD88 death domain (NT), but not the FLAG-MyD88 TIR domain (CT) partially suppresses the interaction between Beclin 1 and Bcl-2. The indicated constructs were transfected into RAW 267.4 cells for 24 h, and then anti-Bcl-2 antibodies were used to immunoprecipitate Bcl2. Co-immunoprecipitated Beclin 1 was detected by immunoblotting. The indicated proteins in cell lysates were detected by immunoblotting. F, overexpression of Bcl-2 attenuates the association of Beclin 1 with MyD88 and Trif. Following transfection of the Bcl-2 expression vector cell lysates were subjected to immunoprecipitation with anti-Beclin 1 antibodies. The resulting immunoprecipitates and the corresponding cell lysates were analyzed by immunoblotting with the indicated antibodies. G, FLAG-tagged Beclin 1 L116A has a decreased association with Trif, MyD88, and Bcl2. Beclin 1 or Beclin 1 L116A was transfected into RAW 267.4 cells and 24 h later immunoprecipitations were performed with anti-FLAG antibodies. The immunoprecipitates were analyzed by immunoblotting with the indicted antibodies. The above results come from one of three independent experiments that gave similar results.