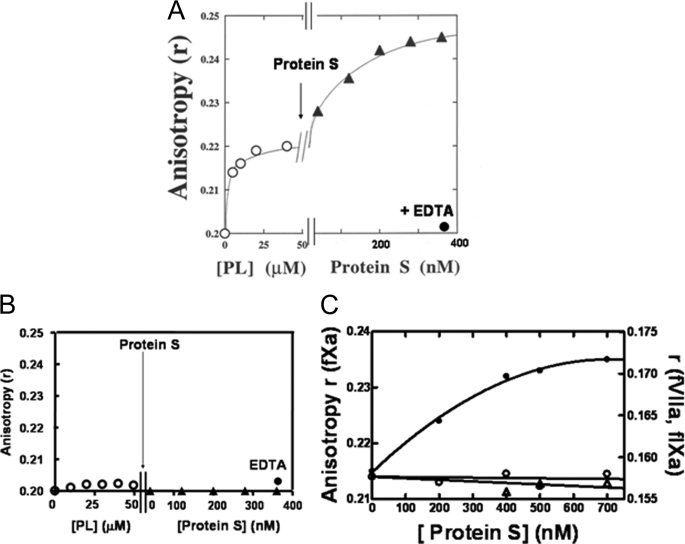
FIGURE 1.
Interaction of protein S with DEGR-fXai in the presence of phospholipid vesicles. Samples containing DEGR-fXai (initially 200 nm) in 50 mm Hepes (pH 7. 4), 150 mm NaCl, 2 mm CaCl2 (buffer A) were titrated with phospholipids (open circles) with either PC/PS (4:1 mole ratio) (A) or 100% PC (B) vesicles prior to the addition of protein S (filled triangles). At the end of the titration, 5 mm EDTA (filled circles) was added to reverse the fluorescence changes. The anisotropy of the dansyl moiety in DEGR-fXai was monitored as described under “Experimental Procedures.” C, factor Xa dependence of dansyl anisotropy change. Three parallel titrations were performed. Equimolar concentrations of DEGR-fXai (filled circles), DEGR-fIXai (open circles), and DEGR-fVIIai (open triangles) (initially 200 nm) were each titrated with PC/PS vesicles as described (31, 32) prior to the addition of protein S. Anisotropy (r) was measured as described above.