Figure 6.
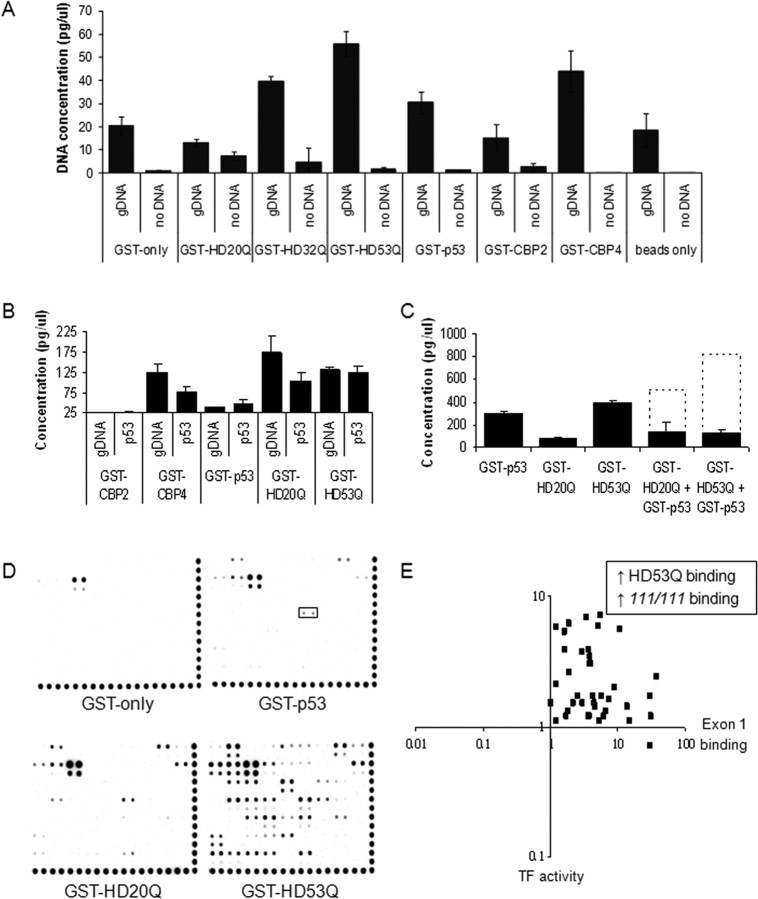
Exon 1 Htt binds nonspecifically to DNA directly in the absence of other proteins in a polyQ-dependent manner. A, Validation of the DIP technique with known DNA interactors [DNA-binding domains of CBP (GST-CBP4, GST-CBP1), GST-p53] and proteins known not to interact with DNA [GST-only, non-DNA-binding domains of CBP (GST-CBP2, GST-CBP5)]. Exon 1 Htt proteins bind significant amounts of genomic DNA, with expanded repeats (GST-HD32Q and GST-HD53Q) typically binding equivalent or more DNA than wild type (HD20Q). Error bars are SEM (n = 4). B, Exon 1 Htt proteins bind a p53 consensus sequence, as does the nonspecific DNA-binding domain of GST-CBP4. C, Exon 1 Htt proteins interfere with GST-p53 DNA binding. The dashed line indicates the predicted amount of genomic DNA binding (estimated by adding the level of binding of GST-p53 and the exon 1 Htt protein species). D, DNA binding by GST fusion proteins on a transcription factor array shows appropriate binding by GST-p53 to its recognition element (boxed area) in addition to some ectopic binding, some of which is explained through GST-only. The GST-HD20Q binding pattern resembles that GST-p53; however, the polyQ repeat expansion in GST-HD53Q potentiates DNA binding. Binding levels were not normalized, because we assayed the presence/absence of binding only. E, Comparing GST–protein binding ratios (HD53Q/GST-HD20Q) with transcription factor activity in cell lines (STHdh111/111/STHdh7/7) shows that transcription factor recognition elements with more binding in HD conditions are those bound by pure mutant exon 1 Htt in vitro (Table 2).