Figure 7.
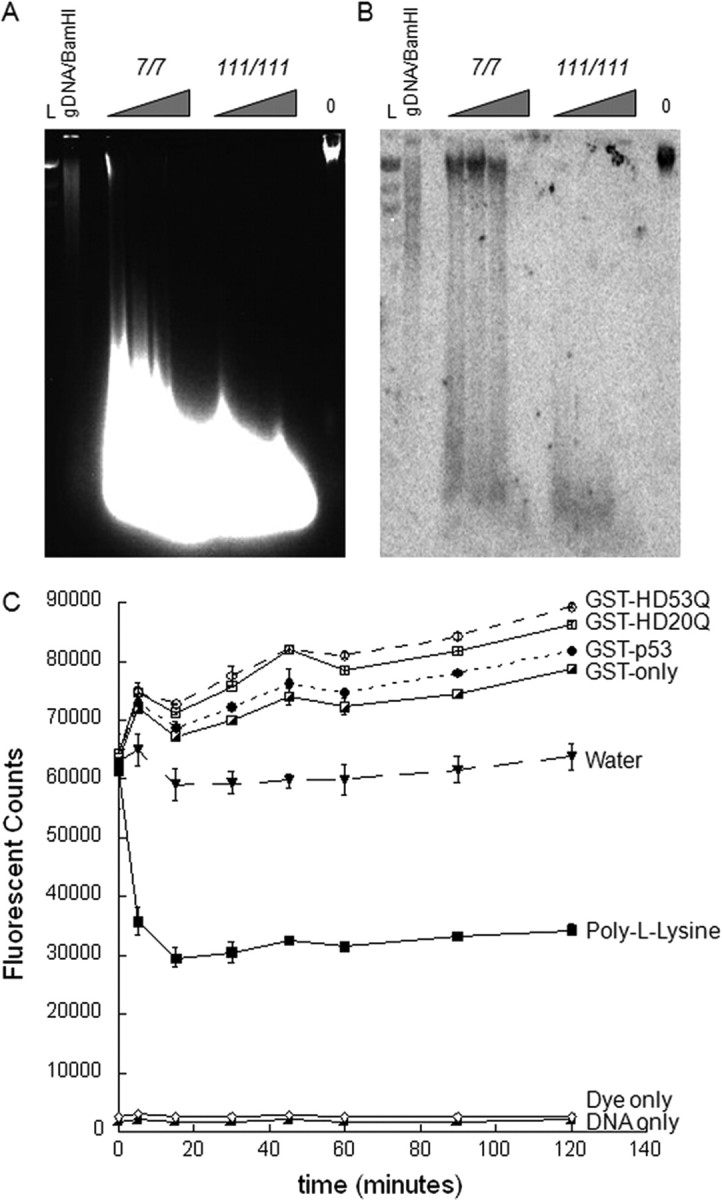
Mutant Htt may contribute to a more open DNA conformation. A, Micrococcal nuclease assay on STHdh7/7 and STHdh111/111 cell lines show more digestion in the mutant Htt cell lines than the wild-type cell lines, which suggests a more open conformation of DNA, facilitating access of the enzyme. Triangles represent amounts of titrated MNase enzyme (from 2.5 to 12.5 units). Also shown is control genomic DNA from STHdh111/111 cell lines with 0 units of MNase enzyme. L is the λHindIII DNA ladder. B, Southern hybridization of a cathepsin H (Ctsh)-specific probe to the MNase-digested chromosomal DNA revealed the presence of high-molecular-weight fragments in the STHdh7/7 cell lines only, with levels of radiolabeled probe binding to high-molecular-weight fragment much higher than levels of high-molecular-weight DNA in the native gel. In contrast, the Ctsh probe hybridized only to low-molecular-weight fragments in the homozygote STHdh111/111 cell lines, suggesting a more open chromatin conformation accessible to digestion by MNase. Probes specific for other genes have demonstrated a similar pattern (data not shown). C, Exon 1 Htt exerts distinct polyQ-dependent effects on DNA conformation, as assayed by DNA–YOYO-1 fluorescence. Addition of 0.5 μm GST fusion proteins increases the YOYO-1 fluorescent signal, which corresponds to a more open DNA conformation. We observed more fluorescence with addition of GST-p53 than GST-only, as expected. Furthermore, addition of the mutant Htt protein GST-HD53Q induces more fluorescence than the wild-type GST-HD20Q. Quenching of the fluorescence by poly-l-lysine, a DNA condensing agent, confirms the assay. Data shown in C are a representative experiment from four independent trials. Error bars indicate SEM.
