Abstract
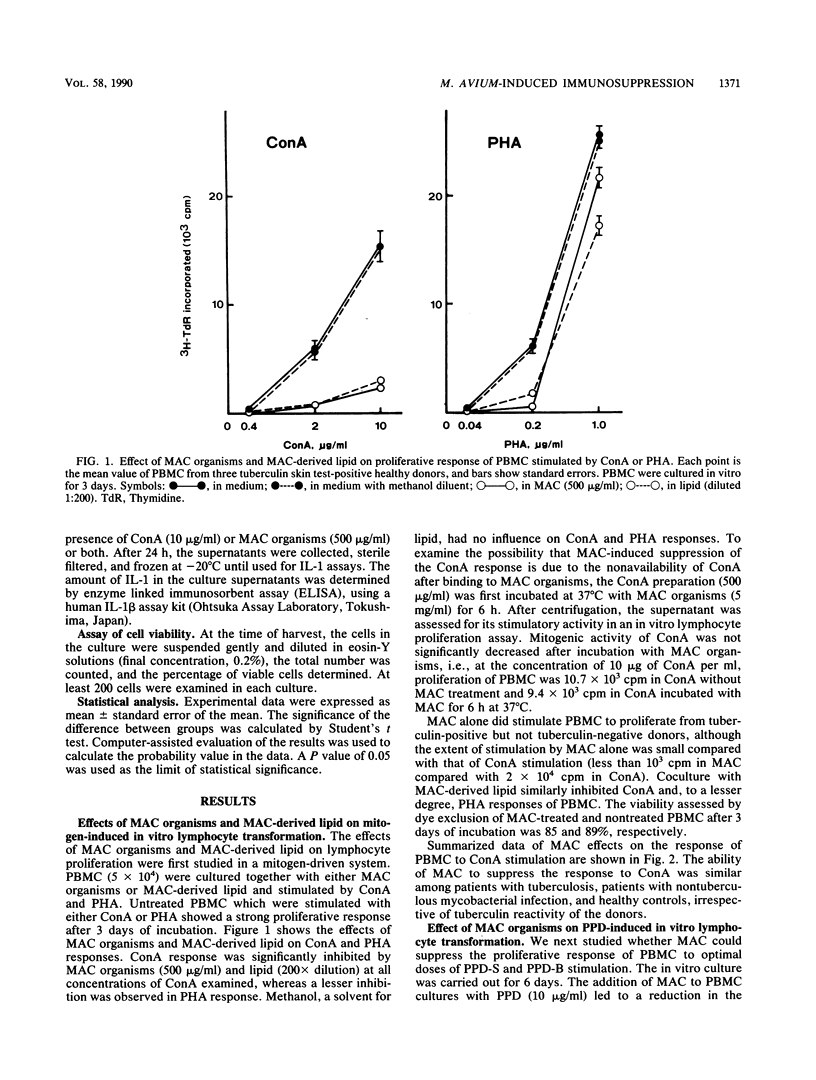
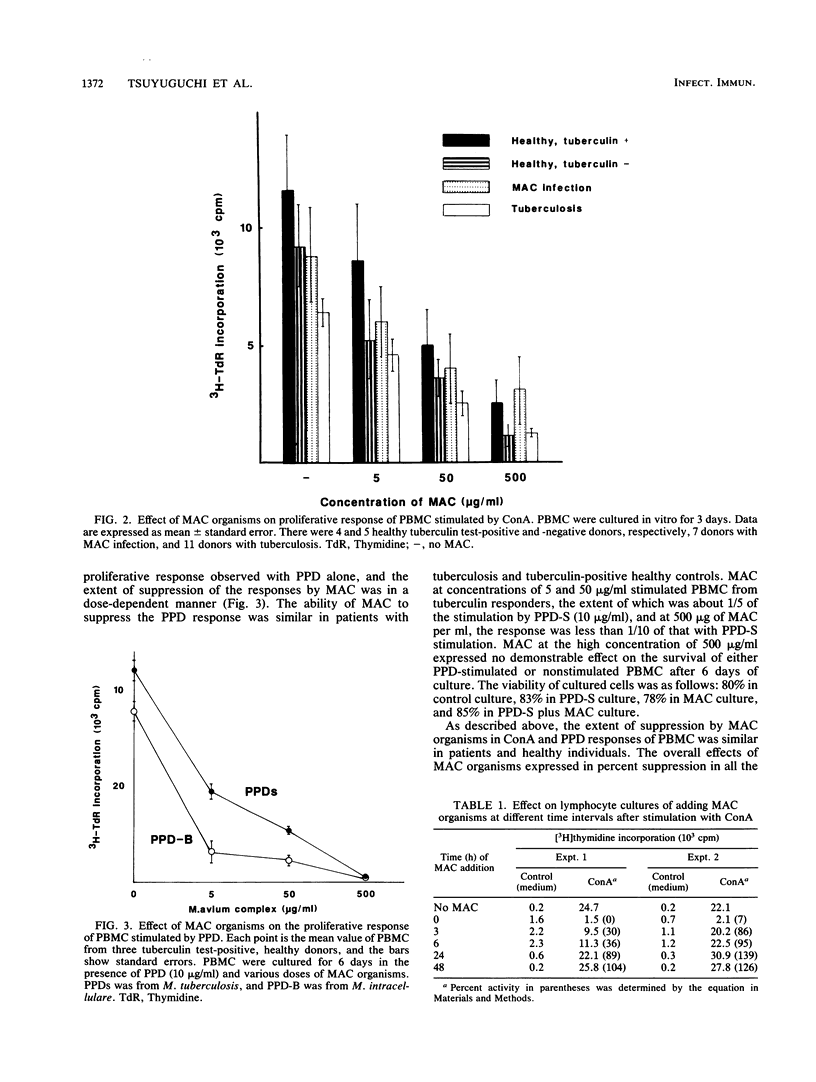
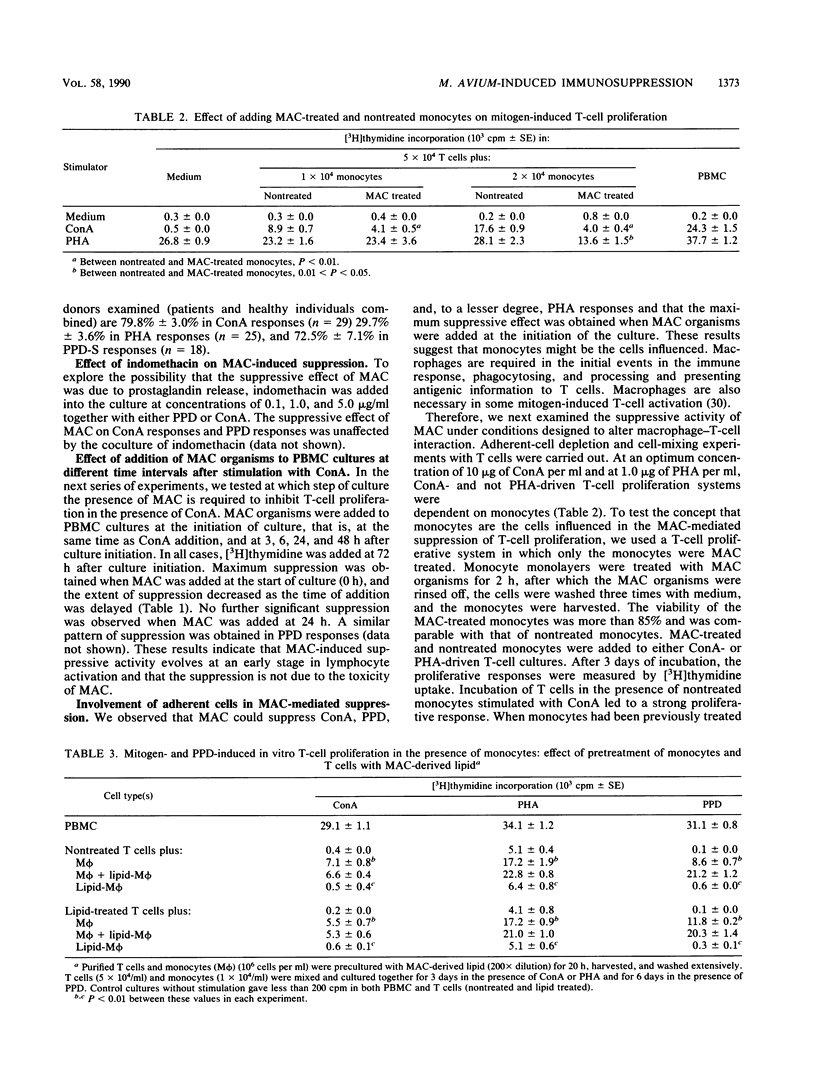
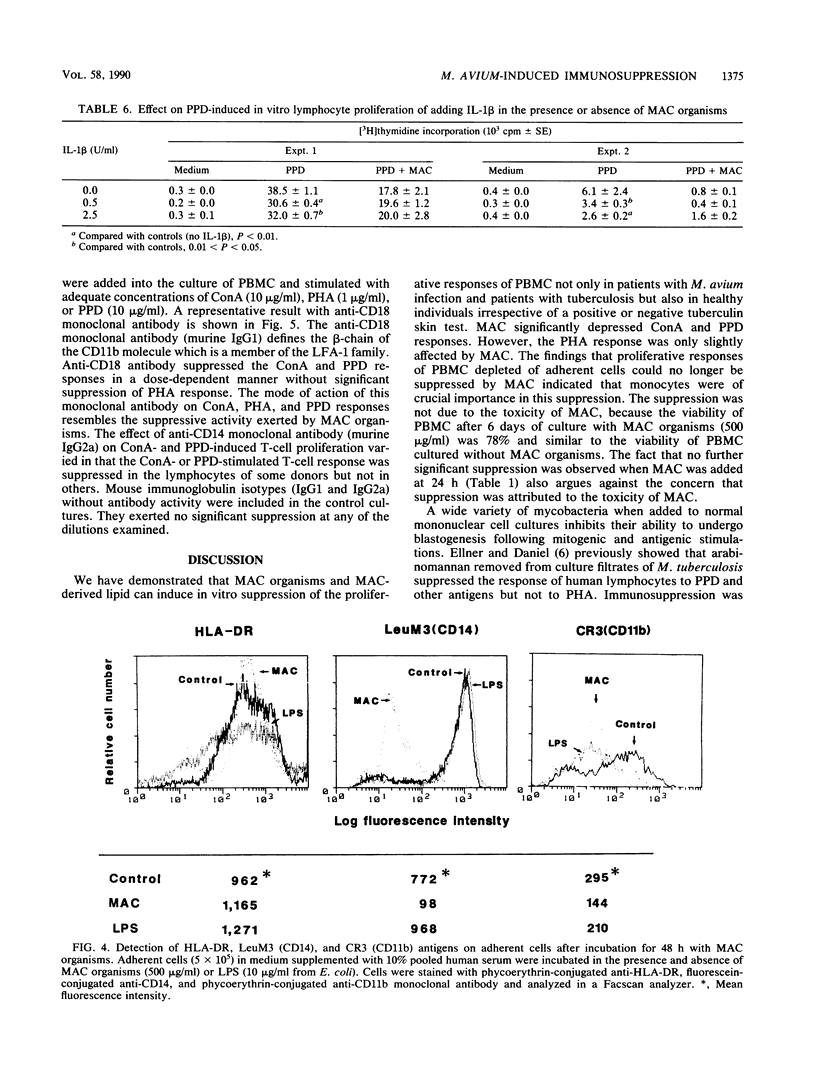
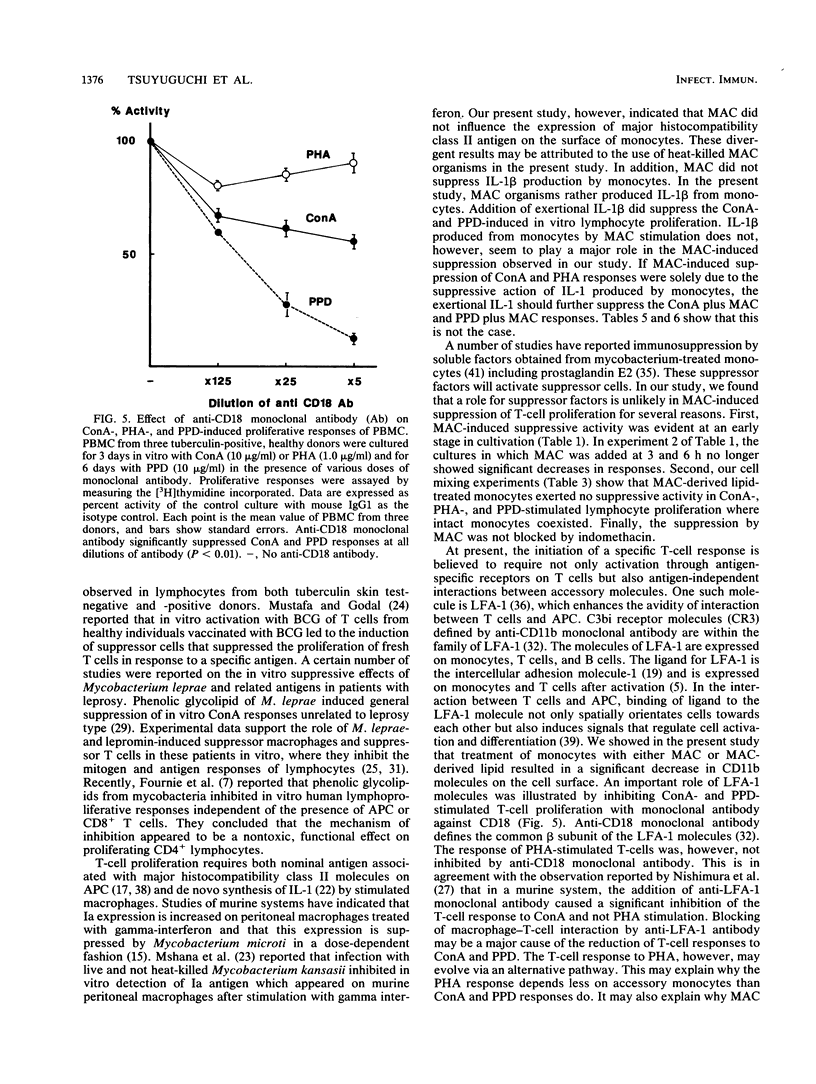
Heat-killed whole Mycobacterium avium-Mycobacterium intracellulare complex (MAC) and its lipid component impaired the capacity of human peripheral blood mononuclear cells to proliferate in vitro in response to concanavalin A (ConA), purified protein derivative of tuberculin (PPD), and to a lesser degree, phytohemagglutinin stimulation. Inhibition by MAC was not contingent upon prior exposure of the donor to MAC or other mycobacteria and occurred with lymphocytes from tuberculin-negative as well as -positive subjects. The suppression was not due to the toxicity of MAC. The suppression by MAC was not blocked by indomethacin. Adherent cell depletion and cell mixing experiments with T cells indicated that monocytes and not T cells were a major contributor to the immunosuppression observed. However, neither interleukin-1 production nor the expression of HLA-DR (Ia antigen) by monocytes was suppressed by MAC treatment. On the other hand, treatment of monocytes with MAC or MAC-derived lipid resulted in significant decreases in CD11b, a member of the leukocyte function-associated molecule-1 and LeuM3 (CD14) molecule. Anti-CD18 (beta-chain of the leukocyte function-associated molecule-1 family) monoclonal antibody had suppressive effects on ConA- and PPD- but not phytohemagglutinin-induced in vitro lymphocyte blastogenesis. We suggest that MAC and MAC-derived lipid suppress the ConA- and PPD-induced T-cell proliferations by blocking the expression of accessory molecules on the surfaces of monocytes which might be involved in nonspecific monocyte-T-cell interactions and not by inhibiting either monocyte Ia antigen expression or interleukin-1 production by monocytes.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Bermudez L. E., Young L. S. Tumor necrosis factor, alone or in combination with IL-2, but not IFN-gamma, is associated with macrophage killing of Mycobacterium avium complex. J Immunol. 1988 May 1;140(9):3006–3013. [PubMed] [Google Scholar]
- Blanchard D. K., Michelini-Norris M. B., Friedman H., Djeu J. Y. Lysis of mycobacteria-infected monocytes by IL-2-activated killer cells: role of LFA-1. Cell Immunol. 1989 Apr 1;119(2):402–411. doi: 10.1016/0008-8749(89)90254-2. [DOI] [PubMed] [Google Scholar]
- Brownback P. E., Barrow W. W. Modified lymphocyte response to mitogens after intraperitoneal injection of glycopeptidolipid antigens from Mycobacterium avium complex. Infect Immun. 1988 May;56(5):1044–1050. doi: 10.1128/iai.56.5.1044-1050.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougherty G. J., Murdoch S., Hogg N. The function of human intercellular adhesion molecule-1 (ICAM-1) in the generation of an immune response. Eur J Immunol. 1988 Jan;18(1):35–39. doi: 10.1002/eji.1830180107. [DOI] [PubMed] [Google Scholar]
- Ellner J. J., Daniel T. M. Immunosuppression by mycobacterial arabinomannan. Clin Exp Immunol. 1979 Feb;35(2):250–257. [PMC free article] [PubMed] [Google Scholar]
- Fournie J. J., Adams E., Mullins R. J., Basten A. Inhibition of human lymphoproliferative responses by mycobacterial phenolic glycolipids. Infect Immun. 1989 Nov;57(11):3653–3659. doi: 10.1128/iai.57.11.3653-3659.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gidlund M., Rossi P., Cotran P., Ramstedt U., Wigzell H. In human monocytes a strong correlation exists between expression of the M3 antigen, Fc-mediated phagocytic activity and failure to participate in extracellular antibody-dependent cytotoxicity. Eur J Immunol. 1988 Mar;18(3):477–480. doi: 10.1002/eji.1830180324. [DOI] [PubMed] [Google Scholar]
- Goyert S. M., Ferrero E., Rettig W. J., Yenamandra A. K., Obata F., Le Beau M. M. The CD14 monocyte differentiation antigen maps to a region encoding growth factors and receptors. Science. 1988 Jan 29;239(4839):497–500. doi: 10.1126/science.2448876. [DOI] [PubMed] [Google Scholar]
- Graham I. L., Gresham H. D., Brown E. J. An immobile subset of plasma membrane CD11b/CD18 (Mac-1) is involved in phagocytosis of targets recognized by multiple receptors. J Immunol. 1989 Apr 1;142(7):2352–2358. [PubMed] [Google Scholar]
- Haziot A., Chen S., Ferrero E., Low M. G., Silber R., Goyert S. M. The monocyte differentiation antigen, CD14, is anchored to the cell membrane by a phosphatidylinositol linkage. J Immunol. 1988 Jul 15;141(2):547–552. [PubMed] [Google Scholar]
- Jeevan A., Asherson G. L. Recombinant interleukin-2 limits the replication of Mycobacterium lepraemurium and Mycobacterium bovis BCG in mice. Infect Immun. 1988 Mar;56(3):660–664. doi: 10.1128/iai.56.3.660-664.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julius M. H., Simpson E., Herzenberg L. A. A rapid method for the isolation of functional thymus-derived murine lymphocytes. Eur J Immunol. 1973 Oct;3(10):645–649. doi: 10.1002/eji.1830031011. [DOI] [PubMed] [Google Scholar]
- Kaplan G., Gandhi R. R., Weinstein D. E., Levis W. R., Patarroyo M. E., Brennan P. J., Cohn Z. A. Mycobacterium leprae antigen-induced suppression of T cell proliferation in vitro. J Immunol. 1987 May 1;138(9):3028–3034. [PubMed] [Google Scholar]
- Kaye P. M., Sims M., Feldmann M. Regulation of macrophage accessory cell activity by mycobacteria. II. In vitro inhibition of Ia expression by Mycobacterium microti. Clin Exp Immunol. 1986 Apr;64(1):28–34. [PMC free article] [PubMed] [Google Scholar]
- Kiehn T. E., Edwards F. F., Brannon P., Tsang A. Y., Maio M., Gold J. W., Whimbey E., Wong B., McClatchy J. K., Armstrong D. Infections caused by Mycobacterium avium complex in immunocompromised patients: diagnosis by blood culture and fecal examination, antimicrobial susceptibility tests, and morphological and seroagglutination characteristics. J Clin Microbiol. 1985 Feb;21(2):168–173. doi: 10.1128/jcm.21.2.168-173.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lechler R. I., Norcross M. A., Germain R. N. Qualitative and quantitative studies of antigen-presenting cell function by using I-A-expressing L cells. J Immunol. 1985 Nov;135(5):2914–2922. [PubMed] [Google Scholar]
- Leclerc C., Morin A., Deriaud E., Chedid L. Inhibition of human IL 2 production by MDP and derivatives. J Immunol. 1984 Oct;133(4):1996–2000. [PubMed] [Google Scholar]
- Marlin S. D., Springer T. A. Purified intercellular adhesion molecule-1 (ICAM-1) is a ligand for lymphocyte function-associated antigen 1 (LFA-1). Cell. 1987 Dec 4;51(5):813–819. doi: 10.1016/0092-8674(87)90104-8. [DOI] [PubMed] [Google Scholar]
- Mehra V., Mason L. H., Fields J. P., Bloom B. R. Lepromin-induced suppressor cells in patients with leprosy. J Immunol. 1979 Oct;123(4):1813–1817. [PubMed] [Google Scholar]
- Mizel S. B. Interleukin 1 and T cell activation. Immunol Rev. 1982;63:51–72. doi: 10.1111/j.1600-065x.1982.tb00411.x. [DOI] [PubMed] [Google Scholar]
- Mshana R. N., Hastings R. C., Krahenbuhl J. L. Infection with live mycobacteria inhibits in vitro detection of Ia antigen on macrophages. Immunobiology. 1988 Apr;177(1):40–54. doi: 10.1016/S0171-2985(88)80090-1. [DOI] [PubMed] [Google Scholar]
- Mustafa A. S., Godal T. BCG-induced suppressor T cells optimal conditions for in vitro induction and mode of action. Clin Exp Immunol. 1985 Dec;62(3):474–481. [PMC free article] [PubMed] [Google Scholar]
- Nath I., Van Rood J. J., Mehra N. K., Vaidya M. C. Natural suppressor cells in human leprosy: the role of HLA-D-identical peripheral lymphocytes and macrophages in the in vitro modulation of lymphoproliferative responses. Clin Exp Immunol. 1980 Nov;42(2):203–210. [PMC free article] [PubMed] [Google Scholar]
- Nathan C. F., Murray H. W., Wiebe M. E., Rubin B. Y. Identification of interferon-gamma as the lymphokine that activates human macrophage oxidative metabolism and antimicrobial activity. J Exp Med. 1983 Sep 1;158(3):670–689. doi: 10.1084/jem.158.3.670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura T., Yagi H., Sato N., Ohta S., Hashimoto Y. The role of lymphokine-activated cell-associated antigen. III. Inhibition of T-cell activation by monoclonal killer-blocking antibody. Cell Immunol. 1987 Jun;107(1):32–39. doi: 10.1016/0008-8749(87)90263-2. [DOI] [PubMed] [Google Scholar]
- Pabst M. J., Gross J. M., Brozna J. P., Goren M. B. Inhibition of macrophage priming by sulfatide from Mycobacterium tuberculosis. J Immunol. 1988 Jan 15;140(2):634–640. [PubMed] [Google Scholar]
- Prasad H. K., Mishra R. S., Nath I. Phenolic glycolipid-I of Mycobacterium leprae induces general suppression of in vitro concanavalin A responses unrelated to leprosy type. J Exp Med. 1987 Jan 1;165(1):239–244. doi: 10.1084/jem.165.1.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenstreich D. L., Farrar J. J., Dougherty S. Absolute macrophage dependency of T lymphocyte activation by mitogens. J Immunol. 1976 Jan;116(1):131–139. [PubMed] [Google Scholar]
- Salgame P. R., Mahadevan P. R., Antia N. H. Mechanism of immunosuppression in leprosy: presence of suppressor factor(s) from macrophages of lepromatous patients. Infect Immun. 1983 Jun;40(3):1119–1126. doi: 10.1128/iai.40.3.1119-1126.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Madrid F., Nagy J. A., Robbins E., Simon P., Springer T. A. A human leukocyte differentiation antigen family with distinct alpha-subunits and a common beta-subunit: the lymphocyte function-associated antigen (LFA-1), the C3bi complement receptor (OKM1/Mac-1), and the p150,95 molecule. J Exp Med. 1983 Dec 1;158(6):1785–1803. doi: 10.1084/jem.158.6.1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selvaraj P., Plunkett M. L., Dustin M., Sanders M. E., Shaw S., Springer T. A. The T lymphocyte glycoprotein CD2 binds the cell surface ligand LFA-3. 1987 Mar 26-Apr 1Nature. 326(6111):400–403. doi: 10.1038/326400a0. [DOI] [PubMed] [Google Scholar]
- Sibley L. D., Hunter S. W., Brennan P. J., Krahenbuhl J. L. Mycobacterial lipoarabinomannan inhibits gamma interferon-mediated activation of macrophages. Infect Immun. 1988 May;56(5):1232–1236. doi: 10.1128/iai.56.5.1232-1236.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibley L. D., Krahenbuhl J. L. Induction of unresponsiveness to gamma interferon in macrophages infected with Mycobacterium leprae. Infect Immun. 1988 Aug;56(8):1912–1919. doi: 10.1128/iai.56.8.1912-1919.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springer T. A., Dustin M. L., Kishimoto T. K., Marlin S. D. The lymphocyte function-associated LFA-1, CD2, and LFA-3 molecules: cell adhesion receptors of the immune system. Annu Rev Immunol. 1987;5:223–252. doi: 10.1146/annurev.iy.05.040187.001255. [DOI] [PubMed] [Google Scholar]
- Tsuyuguchi I., Shiratsuchi H., Okuda Y., Yamamoto Y. An analysis of in vitro T cell responsiveness in nontuberculous mycobacterial infection. Chest. 1988 Oct;94(4):822–829. doi: 10.1378/chest.94.4.822. [DOI] [PubMed] [Google Scholar]
- Unanue E. R., Allen P. M. The basis for the immunoregulatory role of macrophages and other accessory cells. Science. 1987 May 1;236(4801):551–557. doi: 10.1126/science.2437650. [DOI] [PubMed] [Google Scholar]
- Wadee A. A., Mendelsohn D., Rabson A. R. Characterization of a suppressor cell-activating factor (SCAF) released by adherent cells treated with M. tuberculosis. J Immunol. 1983 May;130(5):2266–2270. [PubMed] [Google Scholar]
- Wadee A. A., Sher R., Rabson A. R. Production of a suppressor factor by human adherent cells treated with mycobacteria. J Immunol. 1980 Sep;125(3):1380–1386. [PubMed] [Google Scholar]
- van Noesel C., Miedema F., Brouwer M., de Rie M. A., Aarden L. A., van Lier R. A. Regulatory properties of LFA-1 alpha and beta chains in human T-lymphocyte activation. Nature. 1988 Jun 30;333(6176):850–852. doi: 10.1038/333850a0. [DOI] [PubMed] [Google Scholar]


