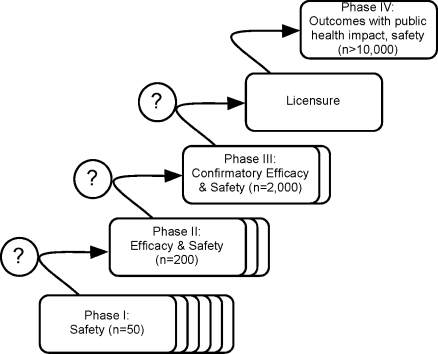
Figure 2. Simplified Outline of the Iterative Process of the Clinical Development of New Antimalarial Candidate Drugs or Drug Combinations.
Phase III trials are designed to provide pivotal efficacy and safety data for obtaining regulatory approval. The indicated numbers of study participants are approximations of the magnitude of required total sample sizes in different transmission settings.