Abstract
Chitinolytic microflora may contribute to biological control of plant-parasitic nematodes by causing decreased egg viability through degradation of egg shells. Here, the influence of Lysobacter enzymogenes strain C3 on Caenorhabditis elegans, Heterodera schachtii, Meloidogyne javanica, Pratylenchus penetrans, and Aphelenchoides fragariae is described. Exposure of C. elegans to L. enzymogenes strain C3 on agar resulted in almost complete elimination of egg production and death of 94% of hatched juveniles after 2 d. Hatch of H. schachtii eggs was about 50% on a lawn of L. enzymogenes strain C3 on agar as compared to 80% on a lawn of E. coli. Juveniles that hatched on a lawn of L. enzymogenes strain C3 on agar died due to disintegration of the cuticle and body contents. Meloidogyne javanica juveniles died after 4 d exposure to a 7-d-old chitin broth culture of L. enzymogenes strain C3. Immersion of A. fragariae, M. javanica, and P. penetrans juveniles and adults in a nutrient broth culture of L. enzymogenes strain C3 led to rapid death and disintegration of the nematodes. Upon exposure to L. enzymogenes strain C3 cultures in nutrient broth, H. schachtii juveniles were rapidly immobilized and then lysed after three days. The death and disintegration of the tested nematodes suggests that toxins and enzymes produced by this strain are active against a range of nematode species.
Keywords: Aphelenchoides fragariae, biological control, Caenorhabditis elegans, chitinase-producing bacterium, Heterodera schachtii, Lysobacter enzymogenes, lytic, Meloidogyne javanica, Pratylenchus penetrans, nematode management, plant-parasitic nematodes
The egg shell of nematodes contains chitin (Maggenti, 1981; Bird and Bird, 1991), and it is presumed that exposing eggs to chitinase can disrupt egg hatch. Both chitinase and chitinase-producing bacteria have been considered for use in reducing numbers of plant-parasitic nematodes in soil (Spiegel et al., 1986, 1987; Mercer et al., 1992; Cronin et al., 1997; Chen et al., 1999).
The genus Lysobacter is in the family Xanthomonadaceae (γ-subdivision of Proteobacteria) and is a genus of gliding and non-fruiting bacteria (Christensen and Cook, 1978; Christensen, 1984). Lysobacter species are typically found in soil and water habitats and are characterized by having gliding motility, high G + C content, and lytic activity against other microorganisms (Christensen and Cook, 1978). Lysobacter enzymogenes degrades chitin and produces lipases and proteases (Katznelson et al., 1964; Christensen and Cook, 1978; Sullivan et al., 2003). “Myxobacteria” strains, which were later placed in L. enzymogenes (Christensen and Cook, 1978), were reported to lyse bacteria-feeding nematodes but not the stylet-bearing nematodes Aphelenchus avenae and Heterodera trifolii (Katznelson et al., 1964); the lytic activity, however, was not investigated further. Lysobacter enzymogenes strain C3, which was repositioned from Stenotrophomonas maltophilia (Sullivan et al., 2003), was found to be effective in the biological control of several fungal pathogens (Zhang and Yuen, 1999; Yuen and Zhang, 2001; Yuen et al., 2001). Suppression of fungal pathogens by L. enzymogenes strain C3 was attributed in part to chitinase production (Zhang and Yuen, 2000a, 2000b; Zhang et al., 2001). The research described here was initiated to investigate the effect of L. enzymogenes strain C3 on nematode eggs because the bacteria produce chitinases and other potentially antagonistic enzymes and compounds. Our observations led to an assessment of effects on vermiform stages of different nematode species.
Here we report on the effects of L. enzymogenes strain C3 on the reproduction and viability of the bacteria-feeding nematode Caenorhabditis elegans. Caenorhabditis elegans has been used as a model system to identify bacterial virulence factors (Kurz and Ewbank, 2000). We also examined the influence of L. enzymogenes strain C3 on the survival of egg and juvenile stages of economically important plant-parasitic nematodes, including the sugar-beet cyst nematode Heterodera schachtii, root-knot nematode Meloidogyne javanica, root-lesion nematode Pratylenchus penetrans, and foliar nematode Aphelenchoides fragariae.
Materials and Methods
Bacteria: A spontaneous rifampicin-resistant derivative of L. enzymogenes strain C3 was used in these experiments (Giesler and Yuen, 1998). Escherichia coli strain OP50, the uracil auxotroph used as food for rearing C. elegans, was obtained from the Caenorhabditis Genetic Center (CGC), University of Minnesota, St. Paul, and was included as a control treatment. Cultures of both bacteria were maintained as cryopreserved stock suspensions at -80 °C (Stiernagle, 1999). For these experiments, L. enzymogenes strain C3 and E. coli strain OP50 were cultured using two different media, agar Nematode Growth Medium (NGM) (Brenner, 1974) or liquid Nutrient Broth (NB) (Becton, Dickinson and Co., Sparks, MD). Lysobacter enzymogenes strain C3 was also cultured in a chitin broth medium modified from Zhang and Yuen (2000b), which induces chitinase production in strain C3 (Zhang and Yuen, 2000a).
Chitin broth contained (NH4)2SO4 at 1.0 g/liter, MgSO4 7H2O at 0.3 g/liter, and KH2PO4 at 1.4 g/liter. Practical-grade chitin (Sigma-Aldrich, St Louis, MO) was added to the solution at 10 g/liter as a carbon source. Yeast extract solution (0.5 g yeast extract in 50 ml distilled water) (Sigma-Aldrich) was autoclaved separately and added to the sterilized broth to a concentration of 0.5 g/liter.
Bacterial lawns were created on NGM agar plates by pipetting 0.1 ml thawed stock suspensions onto each petri dish and spreading the bacteria with a sterile glass pipet. Broth cultures were prepared by pipetting 0.1 ml thawed stock suspension into 100-ml volumes of sterile broth in 500-ml Erlenmeyer flasks, which were incubated at 26°C with shaking at 125 rpm (Innova 4330 Refrigerated incubator shaker, New Brunswick Scientific, Edison, NJ). Chitin broth cultures of L. enzymogenes strain C3 were inoculated 7 d before the start of an experiment and were sieved through a 25-μm-pore sieve to remove larger chitin particles prior to use.
Nematodes: The wild-type C. elegans strain N2 (Bristol) was obtained from the Caenorhabditis Genetics Center. It was cryopreserved at –80°C, and cultures were initiated for each experiment by pipetting 0.1 ml cryopreserved culture onto NGM agar seeded with OP50. Nematode cultures were incubated in the dark for 3 d, and the freshly laid eggs were transferred onto fresh NGM with OP50. The adults that subsequently developed were used in the experiments.
An isolate of H. schachtii from Half Moon Bay, CA, was maintained on sugarbeets and cabbage in a greenhouse. Cysts were rinsed from roots, floated from soil, and collected on sieves. When eggs were needed, they were removed from cysts by gently tearing open the cyst walls using forceps and immersing the cyst contents in 5% commercial bleach for 3 min. Second-stage juveniles were collected from cysts placed on Baermann funnels for 3 d.
Cultures of A. fragariae and P. penetrans were obtained from the California Department of Food and Agriculture and maintained on carrot discs in jars. Juvenile and adult stages were recovered by adding water to the jar and swirling it around to collect nematodes loosened from the carrot surface. Juveniles of M. javanica were obtained from hydroponics cultures (Lambert et al., 1992). Eggs were obtained by bleach extraction (Barker, 1985) of infected tomato roots obtained from plants grown in greenhouses.
Artificial tap water (ATW) (Greenaway, 1970) was used to rinse nematodes and was included in some of the experiments as a control treatment. Petri dishes and cell culture plates containing bacteria and nematodes were sealed with parafilm (Pechiney Plastic Packaging, Menasha, WI) to limit moisture loss and maintained in a dark incubator at 25°C. Microscopic observations of nematodes were made at ×40 magnification.
Caenorhabditis elegans experiments: Adult C. elegans from NGM-E. coli OP50 cultures were placed in filter-sterilized tap water on NGM to reduce bacteria adhering to the nematode cuticle. A young or gravid adult nematode was then transferred onto NGM on which C3 had been cultured for 1, 3, or 5 d. Using a separate set of L. enzymogenes strain C3 cultures on NGM, bacterial numbers were determined by rinsing bacterial cells off the agar surface with ATW and plating dilutions of the rinse on NGM. Populations were expressed as log10 colony-forming units (CFU) per plate. Caenorhabditis elegans were also transferred to 1-, 3- and 5-d-old NGM cultures of E. coli OP50 as the controls. The development, reproduction, and survival of C. elegans were determined daily. Nematode body length was measured to assist in the identification of developmental stages of C. elegans. Each treatment was replicated three times, and the experiment was repeated
A separate set of experiments was conducted as a comparative control for confirming the effects of commercial chitinase on C. elegans. Chitinase (EC 3.2.1.14) from Serratia marcescens was obtained from Sigma-Aldrich (St Louis, MO). Chitinase prepared in 0.05 M potassium phosphate buffer was added after the autoclaved NGM agar was allowed to cool to 55°C so that the chitinase would remain stable (Brurberg et al., 1996). Final chitinase concentrations were 0, 0.021, and 0.21 units/ml. Each agar-chitinase mix was pipetted into wells (0.8 ml/well) of 48-well cell-culture plates and into 6-cm-diam. petri dishes (2 ml/dish), and the hardened agar was inoculated with E. coli OP50. In one experiment, one newly hatched juvenile of C. elegans was transferred into each of 12 replicate wells in a cell-culture plate containing 1-d-old cultures of E. coli OP50. The plates were incubated at 24°C ± 1°C. The nematodes were examined daily and the juveniles counted over 7 d. When the progeny that arose from the original nematode placed in a well reached maturity, the original nematode was transferred into a fresh well containing the same chitinase concentration. In this manner, the total number of first-generation progeny from the original nematode could be determined. In another experiment, a juvenile nematode was placed in a petri dish containing a 1-d-old culture of OP50. There were three replicate cultures per chitinase concentration. These were incubated at 20°C ± 1°C for 7 d without any nematodes being removed. Progeny production and population numbers of C. elegans were assessed by suspending the nematodes on the plate in ATW, diluting the suspension to less than 200 nematodes/ml, and counting the nematodes in 1 ml of a subsample using a dissecting microscope.
Meloidogyne javanica in C3 chitin broth cultures: The influence of L. enzymogenes strain C3 grown in chitin broth on juveniles of M. javanica was evaluated with ATW as the control treatment. Aliquots of 0.9 ml of a 7-d-old L. enzymogenes strain C3 culture and ATW were added to wells of 48-well cell-culture plates. There were eight replicate wells for each treatment. Nematodes were added to each well in a 0.1 ml ATW suspension containing 23 to 37 nematodes. After the nematodes were incubated in the treatments for 4 d, the contents of each well were removed, diluted with 9.0 ml ATW, and incubated for an additional day in a 60- × 15-mm petri dish. Nematode survival was assessed based on the percentage of nematodes moving. The experiment was performed twice, once with a fresh culture of L. enzymogenes strain C3 and the second time with the same culture of L. enzymogenes strain C3 that had been stored for 16 d at 4°C prior to use.
Plant-parasitic nematodes in C3 NB cultures: These experiments tested the effects of L. enzymogenes strain C3 on various plant-parasitic nematodes by exposing nematodes to the following treatments: ATW; sterile NB; NB culture of E. coli OP50; NB culture of L. enzymogenes strain C3; and NB culture of C3 filtered using a 0.22-μm Millipore filter to remove bacterial cells. Three day old bacterial cultures were used. Experiments were carried out in 24-well cell-culture plates (Falcon), with 0.9 ml aliquots of each treatment dispensed into each of five replicate wells.
Separate experiments were conducted twice for A. fragariae, P. penetrans, and M. javanica juveniles. For the experiments involving vermiform stages, approximately 10 nematodes were transferred into each well. Nematodes were counted daily and scored as active, inactive, or missing. The results of the two experiments were similar, so the data from the two experiments were combined for presentation.
Effects of L. enzymogenes C3 on H. schachtii egg hatch: An experiment was conducted to investigate the influence of L. enzymogenes strain C3 on H. schachtii egg hatch. Eggs suspended in filter-sterilized tap water were pipetted onto the surface of NGM with established 1-d-old lawns of L. enzymogenes C3 or E. coli OP50. One and 2 ml filter-sterilized tap water was added to the NGM cultures on d 0 and 5, respectively, to maintain the moisture level necessary for hatching. The effects of L. enzymogenes strain C3 on eggs and the numbers of eggs hatched were examined 10 d later. Each treatment was replicated three times and the experiment was conducted twice.
Effects of NB concentration on C3 activity: Separate experiments were conducted to evaluate the effects of L. enzymogenes strain C3 on juvenile stages of H. schachtii when the bacterium was grown in full- and half-strength NB. The half-strength medium was included to reduce the growth rate of L. enzymogenes strain C3. This experiment was performed twice, the first time using ATW and 0.5× NB as control treatments. A mixture of antibiotics (50 units penicillin, 0.05 mg streptomycin, and 0.1 mg neomycin, per ml, Sigma Chemical, St. Louis, MO) was added to restrict the growth of contaminating bacteria in NB. C3 was cultured in full-strength and diluted NB for 3 d, at which time 1 ml of each treatment solution was pipetted into wells (five wells per treatment) of a 24-well cell-culture plate. Five H. schachtii J2 were transferred into each well. The number of active, inactive, or missing juveniles in each well was determined daily. This experiment was repeated.
Results
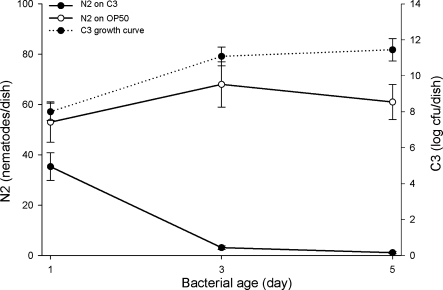
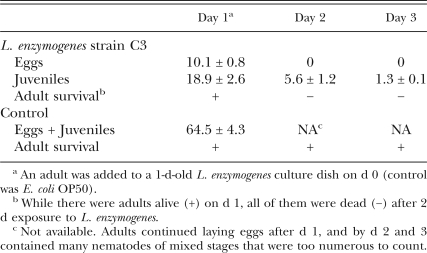
Caenorhabditis elegans experiments: The reproduction of C. elegans was inhibited on NGM cultures of L. enzymogenes C3, and the inhibition was greater in 3- and 5-d-old cultures than in 1-d-old cultures (Fig. 1). Shortly after transfer to L. enzymogenes strain C3 cultures, the nematodes were observed actively feeding with vigorous metacorpal pumping. After 1 d of exposure to C3, however, the rate of metacorpal pumping as observed at ×40 magnification was reduced in adults exposed to C3 compared to adults exposed to OP50. After feeding for 1 d on 1- and 3-d-old C3 cultures, nematode adults produced 35 and 3 progeny/dish, respectively, compared to 53 and 68 in the E. coli OP50 control. On 5-d-old cultures of C3, there was almost no progeny production, compared to 61 progeny produced on E. coli OP50. Numbers of C3 at the beginning of the experiment were approximately 107 in 1-d-old cultures and approximately 1011 in 3- and 5-d-old cultures. Caenorhabditis elegans adults placed on 1-d-old C3 cultures did not produce eggs or survive into the second day (Table 1). None of the juveniles that hatched after d 1 developed into adults, and the number of juveniles present declined over time, such that fewer than two juveniles were alive after 3 d. About 28% of adult C. elegans exposed to L. enzymogenes strain C3 produced progeny internally, the phenomenon of facultative vivipary (Chen and Caswell-Chen, 2003). Nematodes developed normally on the control E. coli OP50 and produced 65 progeny after 1 d and too many progeny to count by d 3 (data not shown).
Fig. 1.
Lysobacter enzymogenes strain C3 (C3) bacterial population growth (without nematodes) curve vs. time (dotted line), and the influence of the age and population density of L. enzymogenes strain C3 on Caenorhabditis elegans strain N2 as compared to C. elegans growing on E. coli OP50 (solid lines). Numbers of nematode eggs and juveniles were counted 1 d after placing a single gravid adult on a 1-, 3-, or 5-d-old C3 bacterial lawn.
Table 1.
Reproduction and survival of Caenorhabditis elegans adults in the presence of Lysobacter enzymogenes strain C3.
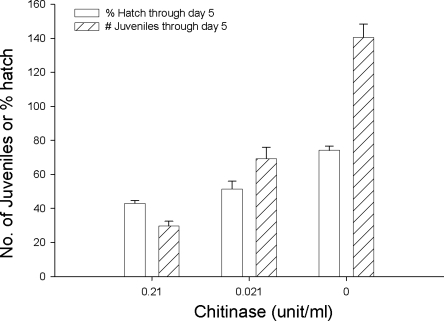
While C. elegans juveniles with E. coli OP50 on chitinase-amended NGM developed, matured, and survived, they produced fewer progeny than those in treatments without chitinase (Fig. 2, Table 2). In the experiment conducted in wells of a cell-culture plate, the percentage of hatched eggs and number of juveniles derived from a single nematode was decreased by chitinase (Fig. 2). A similar effect of chitinase occurred in the petri dish experiment (Table 2).
Fig. 2.
Effects of chitinase on egg production and hatch of Caenorhabditis elegans. One adult was inoculated into a well containing NGM and OP50 E. coli with either 0.21, 0.021, or 0 units of chitinase per ml on d 0. The percentage of egg hatch and number of progeny juveniles per adult through d 5 are plotted. Bars indicate standard error (n = 12).
Table 2.
Effects of exogenous chitinase on reproduction and culture growth (mean ± SE) of Caenorhabditis elegans in vitro on d 7.
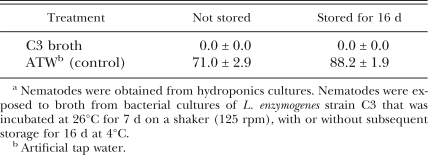
Meloidogyne javanica in C3 chitin broth cultures: No juveniles of M. javanica survived 4 d exposure to L. enzymogenes strain C3 cultured in chitin broth. There was no difference between broth that was freshly collected and broth that had been stored at 4°C for 16 d (Table 3).
Table 3.
Percent survival (mean ± SE) of Meloidogyne javanica juveniles in Lysobacter enzymogenes strain C3a.
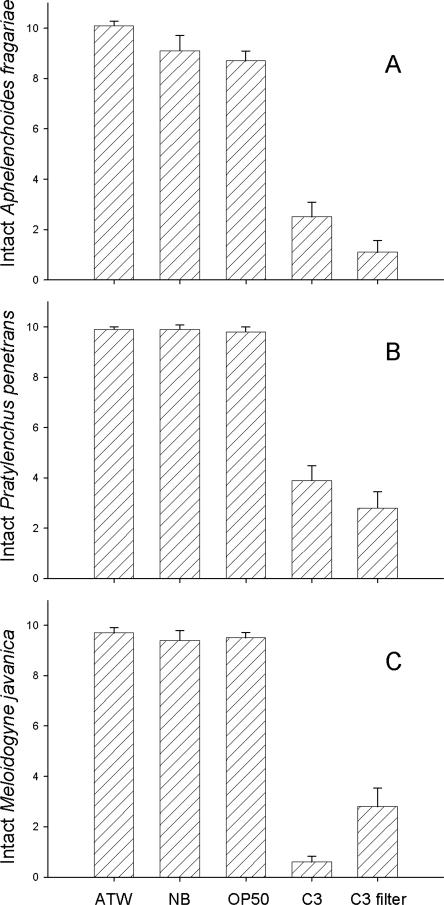
Plant-parasitic nematodes in C3 nutrient broth cultures: Lysobacter enzymogenes strain C3 caused adults and juveniles of A. fragariae and P. penetrans and juveniles of M. javanica to become inactive and then dissolve (Figs. 3,4). A small number of nematodes also became inactive in NB and E. coli OP50 controls as compared to ATW, but none of the nematodes in the control treatments were lysed. The negative effect of NB and E. coli OP50 on activity was likely due to bacterial contamination that inevitably arose in the control wells. In a separate experiment, P. penetrans placed in nutrient broth amended with an antibiotic to prevent bacterial growth remained active longer than those in broth; furthermore, nematodes inactivated by contaminating microorganisms recovered activity when the contaminated broth was diluted 10-fold with water (data not shown).
Fig. 3.
Effects of Lysobacter enzymogenes strain C3 grown in nutrient broth on (A) Aphelenchoides fragariae juveniles and adults; (B) Pratylenchus penetrans juveniles and adults; (C) Meloidogyne javanica juveniles. Data presented are the number of nematodes that were not disintegrated after exposure to the treatments for 2 d. ATW = artificial tap water; NB = nutrient broth; OP50 = E. coli strain OP50; C3 = Lysobacter enzymogenes strain C3; C3 filter = Lysobacter enzymogenes strain C3 culture filtrate. Bars indicate standard error.
Fig. 4.
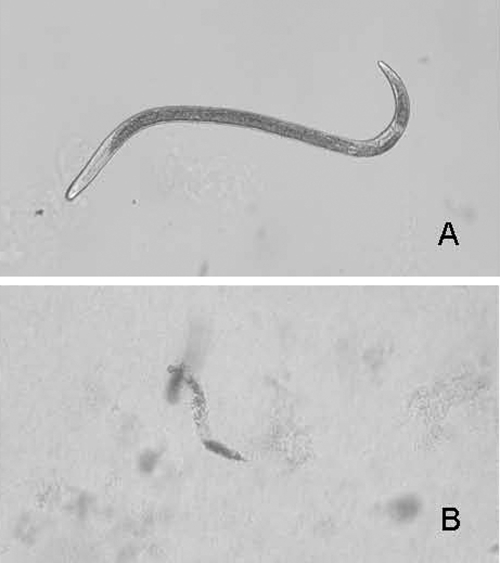
Photomicrograph of Pratylenchus penetrans placed in ATW for 48 hr (A) and the lysed remains of P. penetrans following 48-hr exposure to fluid from a NB culture of Lysobacter enzymogenes strain C3 (B).
Effects of L. enzymogenes C3 on H. schachtii egg hatch: Lysobacter enzymogenes C3 growing on NGM agar reduced hatch of H. schachtii eggs. Hatch of H. schachtii eggs after 10 d exposure to C3 and E. coli OP50 was 49.9% (± 3.7 SE) and 79.3% (± 6.7 SE), respectively. Many of the eggs exposed to C3 appeared abnormal, and the bodies of many H. schachtii juveniles were in poor condition with internal body organization unrecognizable.
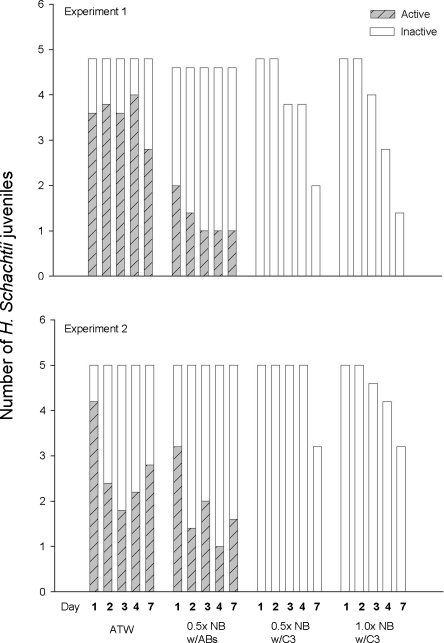
Effects of NB concentration on C3 activity: None of the H. schachtii juveniles placed in full- and half-strength NB cultures of L. enzymogenes C3 were active after 1 d (Fig. 5). In contrast, nematodes in the ATW and the NB + antibiotics control treatments declined in activity during the experiment, but at least 20% of the nematodes remained active after 7 d. Nematodes in the C3 cultures started disintegrating by d 3, with disintegration occurring more rapidly in cultures of C3 in full-strength NB. All of the nematodes in the controls remained intact throughout the experiments.
Fig. 5.
Effects of Lysobacter enzymogenes strain C3 on Heterodera schachtii juveniles. C3 was cultured in the 0.5× and l× NB. The treatments include 0.5× NB with antibiotics (ABs). The number of juveniles that were active or inactive is indicated by hatching. The difference between the full-height columns and the shorter columns indicates the number of juveniles that disintegrated.
Discussion
This research was initiated to investigate the influence of L. enzymogenes strain C3 on nematodes, and the early emphasis was to determine whether it could kill nematode eggs via its production of chitinase. Lysobacter enzymogenes strain C3 reduced the survival of both eggs and juveniles of the bacteria-feeding nematode C. elegans and the plant-parasitic nematode H. schachtii, juveniles of M. javanica, and vermiform stages of P. penetrans and A. fragariae. It suppressed reproduction of C. elegans and killed adult and juvenile nematodes, and many eggs with abnormalities were observed. We discovered that the culture medium was important relative to nematicidal activity because L. enzymogenes strain C3 grown in nutrient broth lysed juveniles of M. javanica and vermiform stages of P. penetrans and A. fragariae. It is evident that L. enzymogenes strain C3 is capable of killing, disintegrating, and dissolving several species of plant-parasitic nematodes in vitro.
Strains of L. enzymogenes studied by Katznelson et al. (1964) killed bacteria-feeding nematodes but not stylet-bearing nematodes. The difference in results between studies could be related to differences among bacterial strains in production of specific metabolites. Strain differences are known to occur in L. enzymogenes, such as in chitinase production (Folman et al., 2003) and induction of plant resistance to fungal pathogens (Kilic-Ekici and Yuen, 2003). The effects of C3 appeared to differ slightly among nematode species, with lysis by C3 appearing most severe on M. javanica and least severe on P. penetrans. This may be due to differences in cuticle structure among nematode species or because our P. penetrans cultures contained both juveniles and adults.
The suppression of nematode activity in control treatments of NB and of E. coli OP50 was likely due to bacterial contamination that always occurred in the control NB wells. Nematodes may lose activity temporarily in contaminated NB and in E. coli OP50 cultures, possibly due to stress, such as anoxia, or toxic compounds produced by contaminating microbes. The loss of nematode activity in C3 cultures may also be influenced in part by anoxia, but nematodes disintegrated only in the presence of C3, indicating that the influence of C3 on nematode activity and viability is the result of toxic and lytic compounds.
In nematodes, chitin occurs as the second layer of the egg shell (Maggenti, 1981; Bird and Bird, 1991), so exposure of eggs to chitinase could be the mechanism for a decrease in egg viability as observed here. Although exogenous chitinase may influence eggs deposited in the environment, it might also influence egg production within the adult. Our observations that chitinase decreases C. elegans hatching and egg production suggest that C3 might possibly exert greater influence on eggs than revealed through assessment of egg hatch alone.
Although chitinase may act on nematode egg shells, chitin is not a constituent of the nematode cuticle. Thus, the lethal effects of L. enzymogenes strain C3 on all C. elegans stages, juveniles of H. schachtii and M. javanica, and vermiform stages of P. penetrans and A. fragariae indicate that other metabolites produced by L. enzymogenes strain C3, such as proteases and lipases (Zhang and Yuen, 2000b), might also be responsible for the toxicityof L. enzymogenes strain C3 to nematodes. The dramatic lysis of vermiform nematodes observed when L. enzymogenes strain C3 was cultured in NB was not observed during culture in chitin broth. This suggests that manipulation of the resources supplied to L. enzymogenes strain C3 can influence its activity against nematodes. The activity of culture filtrates against M. javanica was not lost after storage for 16 d at 4°C, an important consideration if C3 were used to produce bioactive compounds that would be stored prior to use. In summary, the antagonistic effects of L. enzymogenes strain C3 against nematodes in vitro include reduced reproduction and hatching in C. elegans and H. schachtii and, when cultured in nutrient broth, rapid lysis of vermiform stages of H. schachtii, M. javanica, P. penetrans, A. fragariae, and C. elegans. A rich NB culture medium for culturing C3 resulted in a lytic capacity while other media did not, indicating that manipulating bacterial nutrition may influence L. enzymogenes activity against nematodes. It will be important to determine the relevance of the observations described here to the more realistic conditions of nematodes infecting plants in soil.
Footnotes
This research was supported by funding from the USDA (5306–22000–013–01S) and from a faculty research grant at the University of California, Davis. This manuscript is a contribution of the University of Nebraska Agricultural Research Division, Journal Series No. 15008. The C. elegans N2 strain and E. coli strain OP50 used in this work were provided by the Caenorhabditis Genetics Center, which is funded by the NIH National Center for Research Resources (NCRR). Thanks to John Chitambar of the California Department of Food and Agriculture for nematode cultures, Jennifer Haynes and Kristi Sanchez for assistance in the laboratory, and Bruce Jaffee, Harry Kaya, and two anonymous reviewers for helpful suggestions on the manuscript.
This manuscript was edited by Andrea Skantar
Literature Cited
- Barker KR. Nematode extraction and bioassays. In: Barker KR, Carter CC, Sasser JN, editors. An Advanced Treatise on Meloidogyne, Volume II: Methodology. Raleigh, NC: North Carolina State University Graphics; 1985. pp. 19–35. [Google Scholar]
- Bird AF, Bird J. New York: Academic Press; 1991. The structure of nematodes. [Google Scholar]
- Brenner S. The genetics of Caenorhabditis elegans . Genetics. 1974;77:71–94. doi: 10.1093/genetics/77.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brurberg MB, Nes IF, Eijsink VGH. Comparative studies of chitinases A and B from Serratia marcescens . Microbiology. 1996;142:1581–1589. doi: 10.1099/13500872-142-7-1581. [DOI] [PubMed] [Google Scholar]
- Chen J, Abawi GS, Zuckerman BM. Suppression of Meloidogyne hapla and its damage to lettuce grown in a mineral soil amended with chitin and biocontrol organisms. Journal of Nematology. 1999;31:719–725. [PMC free article] [PubMed] [Google Scholar]
- Chen J, Caswell-Chen EP. Why Caenorhabditis elegans adults sacrifice their bodies to progeny. Nematology. 2003;5:641–645. [Google Scholar]
- Christensen P. Genus I. Lysobacter Christensen and Cook 1978. In: Holt JG, editor. Bergey's Manual of Systematic Bacteriology, Vol. 3. Baltimore: Williams & Wilkins; 1984. pp. 2083–2089. [Google Scholar]
- Christensen P, Cook FD. Lysobacter, a new genus of nonfruiting gliding bacteria with a high base ratio. International Journal of Systematic Bacteriology. 1978;28:367–393. [Google Scholar]
- Cronin D, Moenne-Loccoz Y, Dunne C, O'Gara F. Inhibition of egg hatch of the potato cyst nematode Globodera rostochiensis by chitinase-producing bacteria. European Journal of Plant Pathology. 1997;103:433–440. [Google Scholar]
- Folman LB, Postma J, Van Veen JA. Characterization of Lysobacter enzymogenes (Christensen and Cook 1978) strain 3.1T8, a powerful antagonist of fungal diseases of cucumber. Microbiological Research. 2003;158:1–9. doi: 10.1078/0944-5013-00185. [DOI] [PubMed] [Google Scholar]
- Giesler LJ, Yuen GY. Evaluation of Stenotrophomonas maltophilia strain C3 for biocontrol of brown patch disease. Crop Protection. 1998;17:509–513. [Google Scholar]
- Greenaway P. Sodium regulation in the freshwater mollusc Limnaea stagnalis (L.) (Gastropoda: Pulmonata) Journal of Experimental Biology. 1970;53:147–163. doi: 10.1242/jeb.53.1.147. [DOI] [PubMed] [Google Scholar]
- Katznelson HD, Gillespie DG, Cook FD. Studies on the relationships between nematodes and other soil micro-organisms. III. Lytic action of soil myxobacters on certain species of nematodes. Canadian Journal of Microbiology. 1964;10:699–704. doi: 10.1139/m64-089. [DOI] [PubMed] [Google Scholar]
- Kilic-Ekici O, Yuen GY. Induced resistance as a mechanism of biological control by Lysobacter enzymogenes strain C3. Phytopathology. 2003;93:1103–1110. doi: 10.1094/PHYTO.2003.93.9.1103. [DOI] [PubMed] [Google Scholar]
- Kurz CL, Ewbank JJ. Caenorhabditis elegans for the study of host-pathogen interactions. Trends in Microbiology. 2000;8:142–144. doi: 10.1016/s0966-842x(99)01691-1. [DOI] [PubMed] [Google Scholar]
- Lambert KN, Tedford EC, Caswell EP, Williamson VM. A system for continuous production of root-knot nematode juveniles in hydroponic culture. Phytopathology. 1992;82:512–515. [Google Scholar]
- Maggenti A. New York: Springer-Verlag; 1981. General Nematology. [Google Scholar]
- Mercer CF, Greenwood DR, Grant JL. Effect of plant and microbial chitinases on the eggs and juveniles of Meloidogyne hapla chitwood (Nematoda: Tylenchida) Nematologica. 1992;38:227–236. [Google Scholar]
- Spiegel Y, Chet I, Cohn E. Use of chitin for controlling plant-parasitic nematodes: II. Mode of action. Plant and Soil. 1987;98:337–346. [Google Scholar]
- Spiegel Y, Cohn E, Chet I. Use of chitin for controlling plant-parasitic nematodes: I. Direct effects on nematode reproduction and plant performance. Plant and Soil. 1986;95:87–96. [Google Scholar]
- Stiernagle T. Maintenance of C. elegans . C. elegans: A Practical Approach. In: Hope IA, editor. Oxford: Oxford University Press; 1999. pp. 51–67. [Google Scholar]
- Sullivan RF, Holtman MA, Zylstra GJ, White JF, Kobayashi DY. Taxonomic positioning of two biological control agents for plant diseases as Lysobacter enzymogenes based on phylogenetic analysis of 16S rDNA, fatty acid composition and phenotypic characteristics. Journal of Applied Microbiology. 2003;94:1079–86. doi: 10.1046/j.1365-2672.2003.01932.x. [DOI] [PubMed] [Google Scholar]
- Yuen GY, Steadman JR, Lindgren DT, Schaff D, Jochum C. Bean rust biological control using bacterial agents. Crop Protection. 2001;20:395–402. [Google Scholar]
- Yuen GY, Zhang Z. Control of brown patch using the bacterium Stenotrophomonas maltophilia C3 and culture fluid. International Turfgrass Society Research Journal. 2001;9:742–747. [Google Scholar]
- Zhang Z, Yuen GY. Biological control of Bipolaris sorokiniana on tall fescue by Stenotrophomonas maltophilia strain C3. Phytopathology. 1999;89:817–822. doi: 10.1094/PHYTO.1999.89.9.817. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Yuen GY. Effects of culture fluids and preinduction of chitinase production on biocontrol of Bipolaris leaf spot by Stenotrophomonas maltophilia C3. Biological Control. 2000a;18:277–286. [Google Scholar]
- Zhang Z, Yuen GY. The role of chitinase production by Stenotrophomonas maltophilia strain C3 in biological control of Bipolaris sorokiniana . Phytopathology. 2000b;90:384–389. doi: 10.1094/PHYTO.2000.90.4.384. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Yuen GY, Sarath G, Penheiter AR. Chitinases from the plant disease biocontrol agent, Stenotrophomonas maltophilia C3. Phytopathology. 2001;91:204–211. doi: 10.1094/PHYTO.2001.91.2.204. [DOI] [PubMed] [Google Scholar]