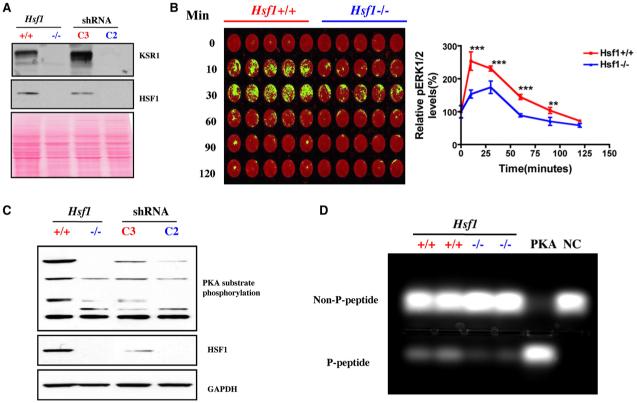
Figure 4. HSF1 Modulates Signal Transduction.
(A) Hsf1-/- and C2-transduced MEFs show remarkably decreased expression of KSR1 protein by immunoblotting. Ponceau red staining was used to confirm equal protein loading.
(B) Hsf1-/- MEFs display blunted ERK activation in response to serum stimulation. Cells were stimulated with 20% FBS for the periods of time indicated, then fixed and stained with phospho-ERK1/2 antibody (green) and the DNA stain TO-PRO-3 iodide (red), which was used to normalize for relative cell number. The plate was scanned, and data are presented as relative phospho-ERK1/2 level by setting values at 0 min as 100% (mean ± SD, n = 5, **p < 0.01; ***p < 0.001; two-way ANOVA).
(C) Hsf1-/- and Hsf1 knockdown cells show markedly reduced phosphorylation of endogenous PKA substrates. Equal amounts of total protein were loaded and probed with antibodies recognizing phospho-(Ser/Thr) PKA substrate, HSF1, and GAPDH, respectively.
(D) Hsf1-/- MEFs possess lower PKA activity. The PKA activity in lysates prepared from Hsf1+/+ and Hsf1-/- MEFs was measured using the classical substrate Kemptide. Reaction mixtures were fractionated by agarose gel electrophoresis to visualize the extent of substrate phosphorylation. PKA, recombinant PKA as positive control. NC, peptide substrate only without lysate or recombinant PKA. The relative amount of phosphorylated peptide in each lane was quantitated by fluorometry.