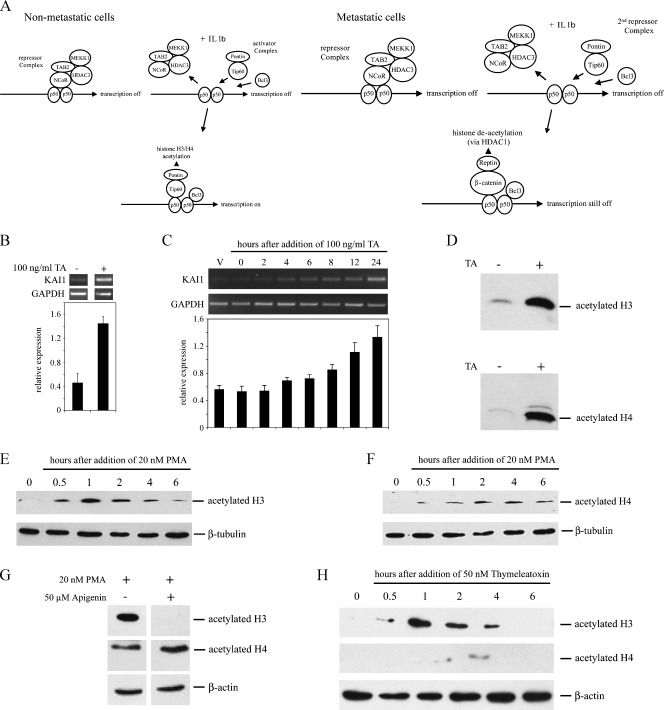
Figure 3.
Trichostatin A and PMA induce acetylation of H3 and H4 histones in LNCaP cells. (A) Schematic of the proposed arrangement of Tip60 or β-catenin containing complexes on the KAI1 promoter, in nonmetastatic and metastatic cancer cells, before and after stimulation with IL-1β. Diagram modified from Kim et al. [14]. (B) Polymerase chain reaction analysis of KAI1 and GAPDH levels in LNCaP treated for 24 hours with DMSO vehicle or with 100 ng/ml trichostatin A in DMSO (TA). Graph shows relative levels of KAI1 (KAI1/GAPDH). Data are means ± SE of at least three independent experiments. (C) Polymerase chain reaction analysis of KAI1 and GAPDH in LNCaP treated for up to 24 hours with 100 ng/ml trichostatin A in DMSO. Vehicle-treated cells (V) were exposed to DMSO only for 24 hours. Graph summarizes mean ± SE from four independent experiments. *P < .05 compared with vehicle-only control. (D) Western blot analysis of acetylated histones H3 and H4, in lysates from LNCaP cells treated for 8 hours with DMSO vehicle (-) or 100 ng/ml trichostatin A (TA; +). (E, F) Western blot analysis of acetylated H3 (E) and H4 (F) histones in lysates from LNCaP cells treated with 20 nM PMA for the indicated times. (G) Western blot analysis for acetylated histones H3 and H4 in LNCaP cells after a 1-hour exposure to 20 nM PMA either without (-) or with (+) 1 hour of pretreatment with 50 µM apigenin. (H) Cells were treated with 50 nM thymeleatoxin for the indicated times, and lysates were used for Western blot analysis of acetylated histones H3 and H4 as described in the Materials and Methods section.