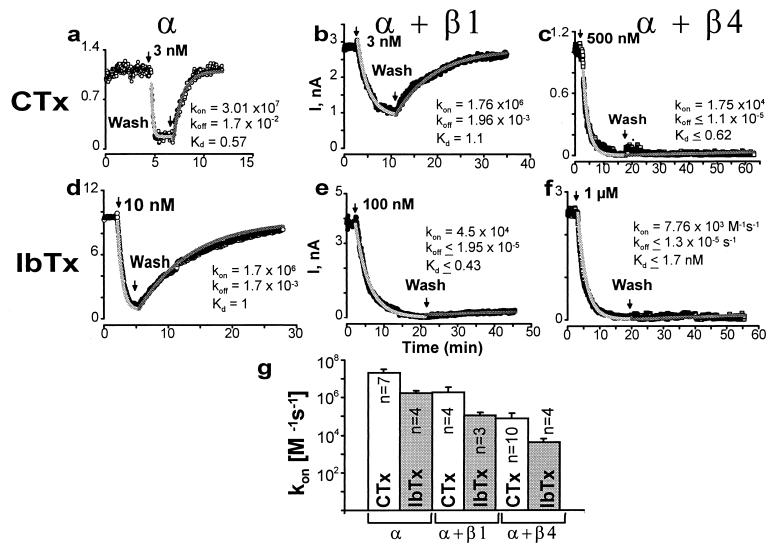
Figure 4.
β4 subunit dramatically slows down CTx and IbTx association and dissociation. Currents in outside-out patches were evoked by repetitive pulses as in Fig. 3. Currents were measured at the end of the pulse and plotted as a function of time; arrows mark the time of toxin application and washout. To attain toxin block in a reasonable time, CTx concentrations were 3 nM for α (a) and α + β1 (b), and 500 nM for α + β4 (c). IbTx concentrations were 10 nM for α (d), 100 nM for α + β1 (e), and 1 μM for α + β4 (f). kon, koff, and Kd values are given in M−1s−1, s−1, and nM, respectively, and were obtained as described in Experimental Procedures. Gray lines are fits to data. (g) Mean kon values (M−1s−1) ± SD (error bars) for CTx and IbTx block in α, α + β1, and α + β4 channels. CTx: kon-α = 2 ± 1 × 107 (n = 7), kon-α+β1 = 1.9 ± 1.8 × 106 (n = 4), and kon-α+β4 = 8 ± 7 × 104 (n = 10); IbTx: kon-α = 2 ± 0.7 × 106 (n = 4), kon-α+β1 = 1.1 ± 0.5 × 105 (n = 3), and kon-α+β4 = 4 ± 2 × 103 (n = 4). n = number of experiments. For both toxins, values for α + β1 and α + β4 were significantly different from those of α subunit (P < 0.005). In two out of seven cases, IbTx block of α + β4 channels had even slower on-rates (not included in mean value).