Figure 3. Assessment of the role of alternative activation regulated by macrophage PPARδ in WAT homeostasis using a co-culture model.
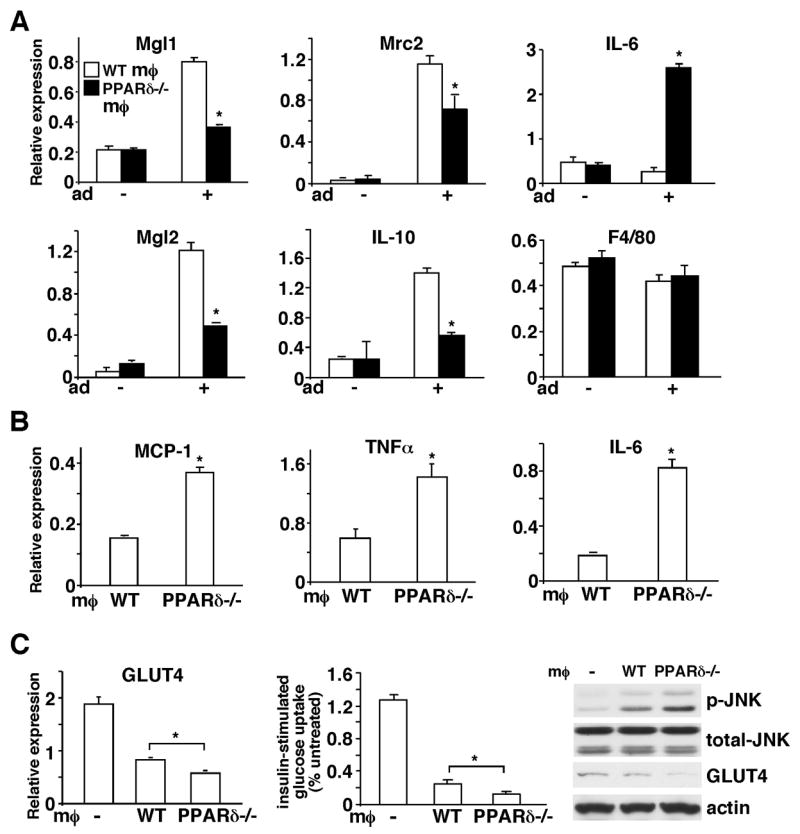
(A) PPARδ plays an important role in macrophage alternative activation induced by adipocyte-derived Th2 cytokines. A co-culture system was established to mimic the WAT microenvironment, in which macrophages were cultured in an insert on a semi-permeable membrane and differentiated 3T3-L1 adipocytes were grown in the lower chamber of the well. Gene expression in macrophages was determined 48 hours after co-culturing with adipocytes by real-time PCR. Mgl1, Mgl2, Mrc2 and IL-10 are M2 markers and F4/80 is a macrophage marker included as a control. The − and + signs indicate without or with adipocytes (Ad) co-culture. (B) Adipocytes co-cultured with PPARδ−/− macrophages express higher levels of pro-inflammatory mediators. Two days after co-culturing with macrophages, adipocytes were harvested and gene expression was determined by real-time PCR. (C) Adipocytes co-cultured with PPARδ−/− macrophages have reduced GLUT4 expression and insulin stimulated glucose uptake. The expression of GLUT4 in control adipocytes (indicated with −) or adipocytes co-cultured with WT or PPARδ−/−macrophages was determined by real-time PCR and Western blotting. The glucose uptake assay was performed using radioactive 2-deoxy-glucose. p-JNK: phospho-JNK; t-JNK: total JNK; MØ: macrophage. Values are expressed as means ± SEM. *p<0.05, comparing WT to PPARδ−/− macrophages.
