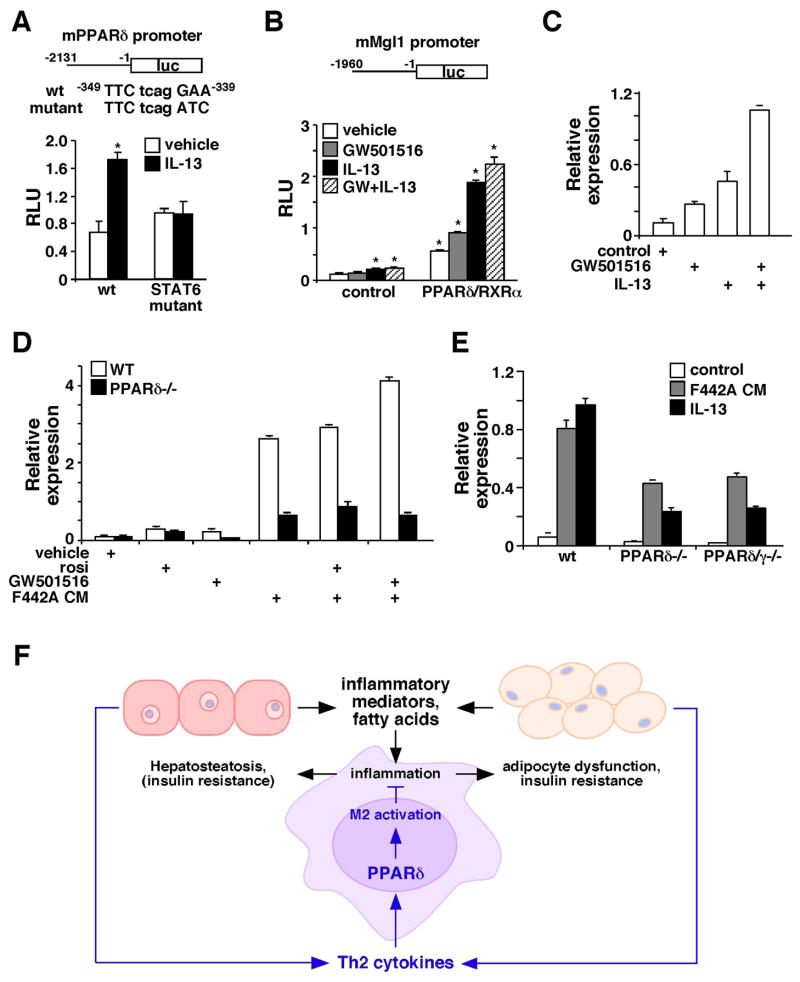
Figure 7. Synergistic regulation of M2 gene expression by IL-13 and PPARδ.
(A) PPARδ promoter activity is induced by IL-13 through a STAT6 binding site. A luciferase (luc) reporter driven by mouse PPARδ promoter (upper panel) was transfected into RAW264.7 cells. IL-13 was given at 40 ng/ml. The transcriptional initiation site was designated as +1 and the putative STAT6 binding site was indicated. STAT6 mutant: PPARδ promoter reporter with the mutated STAT6 binding site; RLU: relative luciferase unit. Values are expressed as means ± SEM. *p<0.05. (B) Mgl1 promoter is regulated by PPARδ and IL-13. Mgl1 promoter reporter was transfected into RAW264.7 cells, together with either the control vector or expression vectors for PPARδ and RXRα. Cells were treated with IL-13 (40 ng/ml) and/or a PPARδ agonist, GW501516 (0.1 μM). *p<0.05, comparing to vehicle treated control. (C) Co-treatment with IL-13 and GW501516 synergistically induces Mgl1 expression in the macrophage. Mgl1 expression was determined 48 hours after treatments by real-time PCR. (D) PPARγ activation could not compensate for PPARδ function in alternative activation. Mgl1 expression was measured in WT and PPARδ−/− macrophages treated with rosiglitazone (rosi, PPARγ agonist, 1μM), GW501516, 3T3 F442A adipocyte CM or the combinations. (E) PPARδ/γ−/− and PPARδ−/− macrophages show a similar reduction in F442A adipocyte CM and IL-13 stimulated Mgl1 expression. (F) Model for the role of macrophage PPARδ in metabolic homeostasis. Macrophages in WAT and the liver are prone to activation by stimulants, such as Th1 cytokines and free fatty acids. As a regulatory mechanism, adipocytes and hepatocytes produce Th2 cytokines to dampen inflammation. The signaling of Th2 cytokines is amplified through the activation of STAT6 and induction of PPARδ. Fatty acids also serve as ligands to activate PPARδ to control the expression of M2 genes as well as genes encoding oxidative metabolism, including PGC-1δ, which is required for STAT6 co-activation. High fat feeding or PPARδ gene deletion disrupts this homeostasis, leading to metabolic dysfunction in WAT and the liver.