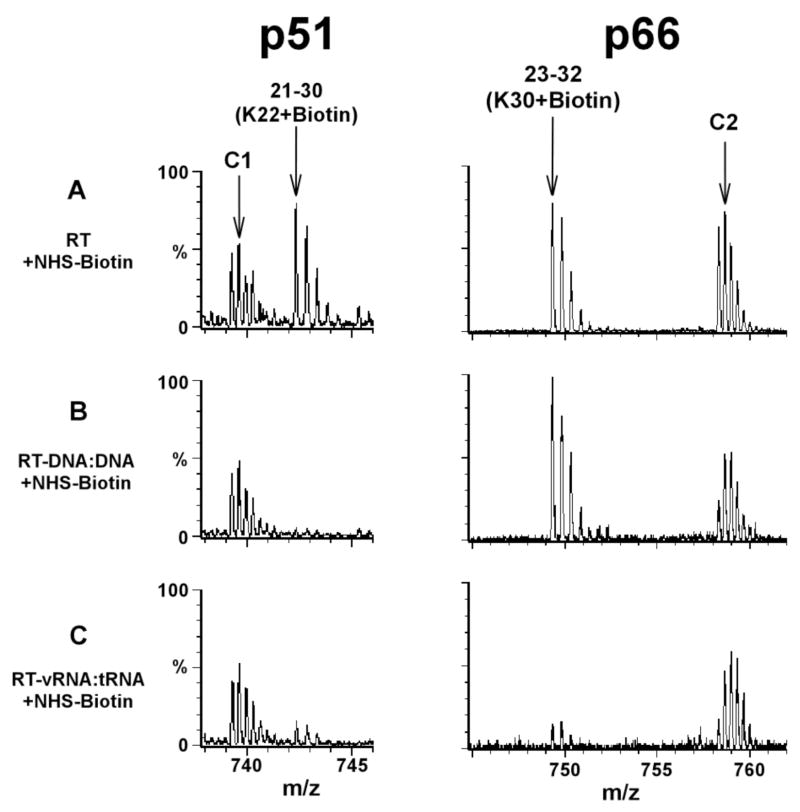
Figure 2.
Mass spectrometric data showing similarity (left panel) and differences (right panel) between HIV-1 RT when complexed with DNA:DNA and a viral RNA:tRNA duplex. RT is comprised of two protein subunits p66 and p51. Treatment of free RT with NHS-biotin resulted in modification of K22 of p51 yielding the peak corresponding to the 21–30 peptide stretch, plus one biotin molecule (A, left panel). In the context of the RT-DNA:DNA (B, left panel) or RT-viral RNA:tRNA complex (C, left panel) this peak was significantly diminished, most likely due to protection by the cognate nucleic acid contacts. The modification of K30 in p66 is shown in the right panel. The 23–32(K30+Biotin) peak persisted in RT-DNA:DNA (B, right panel) but diminished significantly in the RT-viral RNA:tRNA complex (C, right panel). These data indicate that K22 of p51 is coordinating both DNA:DNA and viral RNA:tRNA, while K30 of p66 contacts specifically the viral RNA:tRNA duplex and not DNA:DNA. Unmodified RT peptide peaks C1 and C2 serve as internal controls. Each multiply-charged peptide ion resulted in a clearly resolved peak cluster, indicating monoisotopic resolution in our mass spectrometric analysis. Data adapted from Kvaratskhelia et al. (10)