Fig. 3. Wild-type and the R1441C pathogenic mutant of LRRK2 selectively interact with α/β-tubulin heterodimers.
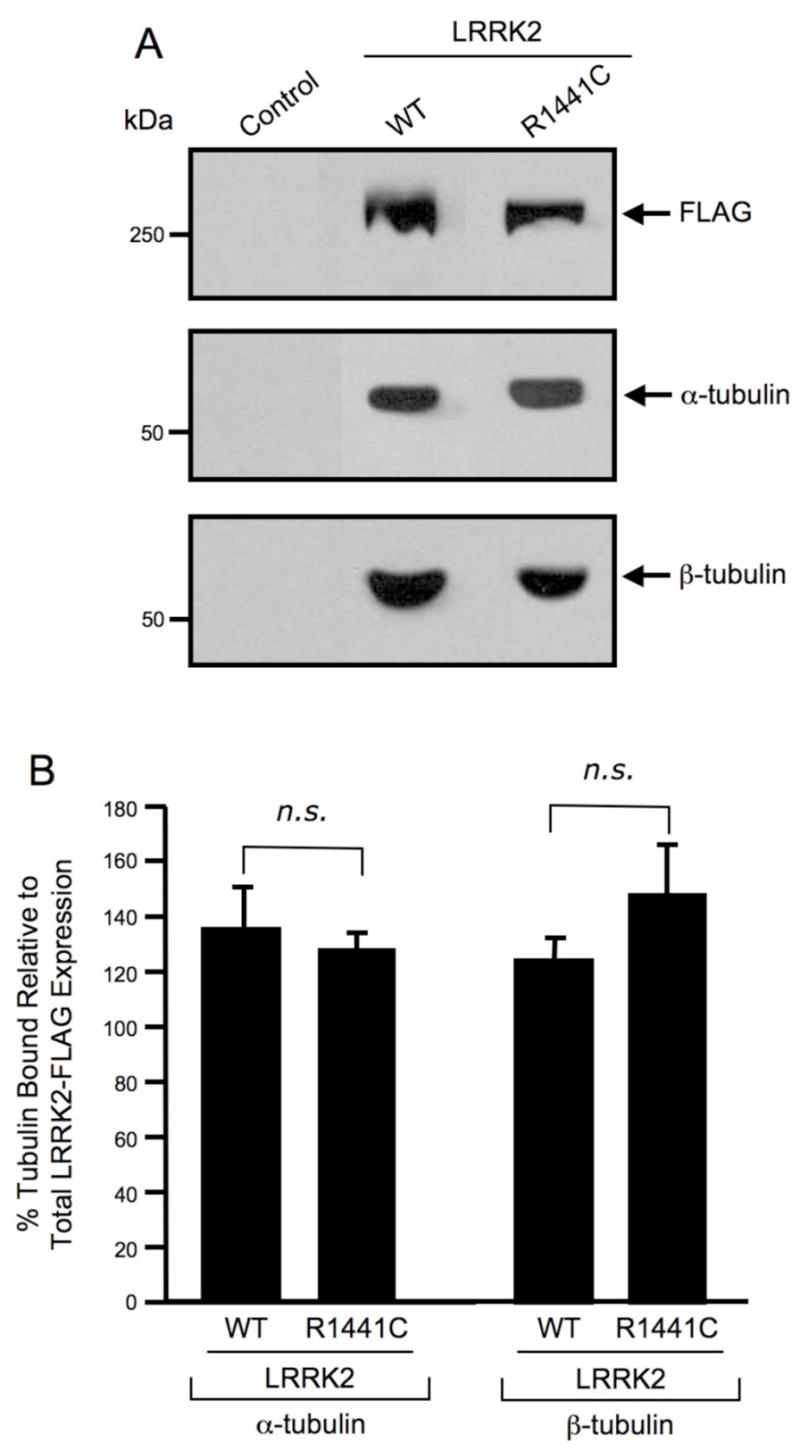
(A) Western blot analysis of LRRK2 co-precipitation assay. FLAG-tagged wild-type and mutant LRRK2 proteins were overexpressed in HEK293T cells and immunoprecipitated with anti-LRRK2 coupled to Dynabeads. Immunoprecipitates were separated by 6% Tris-glycine SDS-PAGE, electrophoretically transferred to PVDF and Western blotted with FLAG, α-tubulin and β-tubulin antibodies. Control refers to untransfected cells. The Western blots shown are representative of three independent experiments. (B) Quantification of Western blot analysis of LRRK2 co-precipitation assay. FLAG, α-tubulin and β-tubulin Western blots were quantified by densitometry and the percentage of α/β-tubulin bound was normalized to LRRK2-FLAG protein expression levels. Error bars represent SEM for three independent experiments. n.s. is non-significant as assessed by a two-tailed unpaired Student’s t-test.
