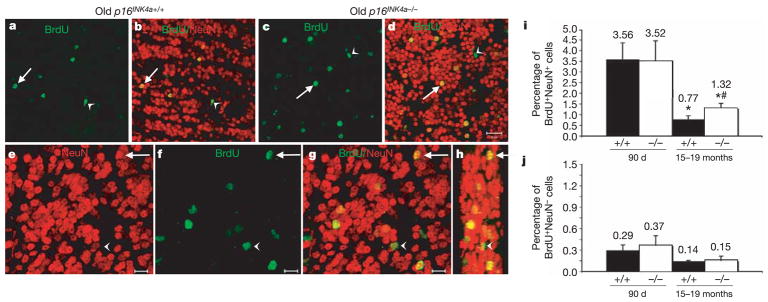
Figure 3. p16INK4a causes age-related declines in olfactory bulb neurogenesis.
a–d, Low-magnification images (scale bar, 20μm) from sagittal sections of the olfactory bulb of old wild-type (a, b; same field of view) and p16INK4a-deficient (c, d; same field of view) mice. Arrows point to new neurons in the granular layer (BrdU+NeuN+) whereas arrowheads point to new non-neuronal cells (BrdU+NeuN−). e–h, Higher magnification images (scale bar, 10μm) from one field of view from an old p16INK4a-deficient mouse. Arrow indicates BrdU+NeuN+ neuron; arrowhead indicates BrdUþNeuN2 non-neuronal cell. Panel h is a three-dimensional, reconstructed side view (80° turn in the z-axis) of panel g. i, Neurogenesis significantly (asterisk, P < 0.05) declined with age (BrdU+NeuN+ neurons as a percentage of all NeuN+ neurons). p16INK4a deficiency did not affect the level of neurogenesis in young mice, but significantly (hash, P = 0.02 relative to old wild-type mice) increased neurogenesis in old mice. j, The frequency of BrdU+NeuN− non-neuronal cells was not significantly affected by p16INK4a deficiency (also as a percentage of NeuN+ neurons). The same trends were observed when the counts were expressed per unit area (not shown). Values are mean ± s.d. from 25 to 30 fields of view per mouse, three mice per treatment.