Figure 3.
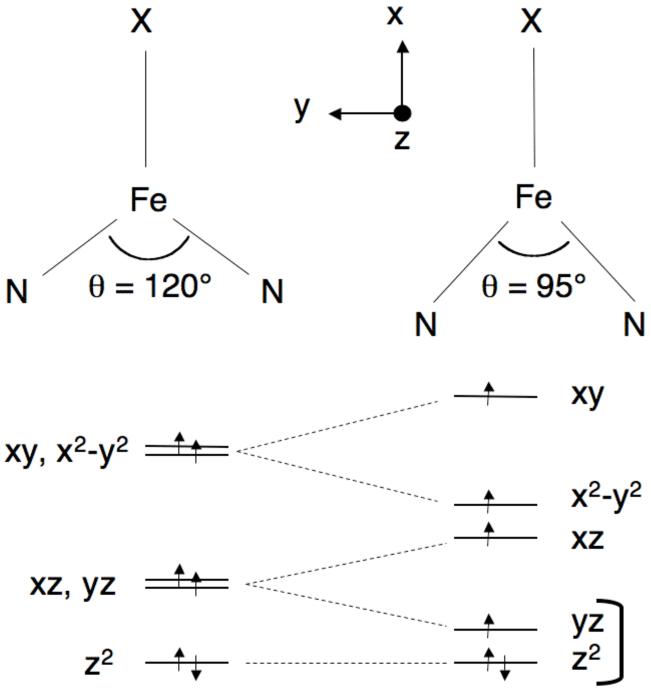
Changes in d orbital energies that result from the 95° bite angle of the β-diketiminate ligand. The yz and z2 orbitals are very close in energy, giving unusual magnetic properties. The xy orbital, which points directly at the N atoms of the β-diketiminate ligand, becomes highest in energy. (In this picture, the z axis is chosen to be out of the plane, rather than the standard axis choice in the C2v point group.)
