Figure 1.
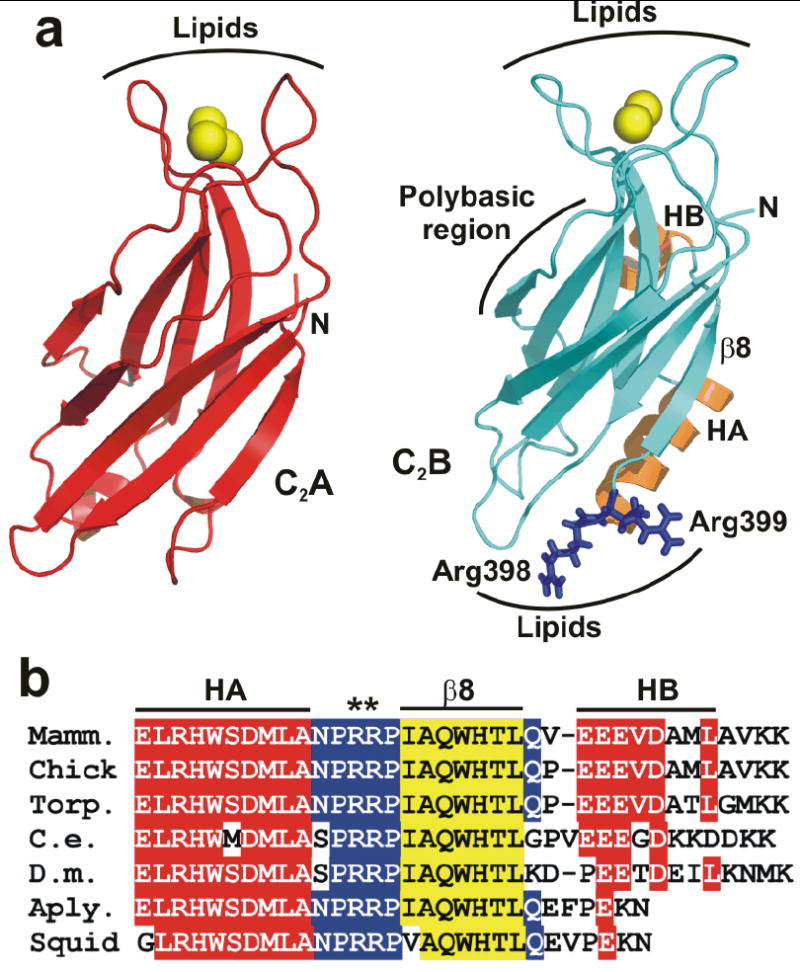
Two highly conserved arginines at the bottom face of the synaptotagmin-1 C2B domain. (a) Ribbon diagrams of the synaptotagmin-1 C2A and C2B domain7, 8 illustrating their structural differences. Ca2+ ions are shown as yellow spheres, helices HA and HB of the C2B domain are colored in orange, and R398 and R399 are shown in blue stick models. Strand β8 of the C2B domain is labeled. The lipid-binding sites of both C2 domains are indicated. The binding site for the SNARE complex is tentatively assumed to be in the polybasic region on one side of the C2B domain β-sandwich17, but note that this region has also been implicated in other interactions, including lipid binding25. (b) Sequence alignment of the C-terminal region of synaptotagmin-1 C2B domains from different species. Conserved residues are colored in red (helices HA and HB), blue (loops) and yellow (strand β8).
