Figure 4.
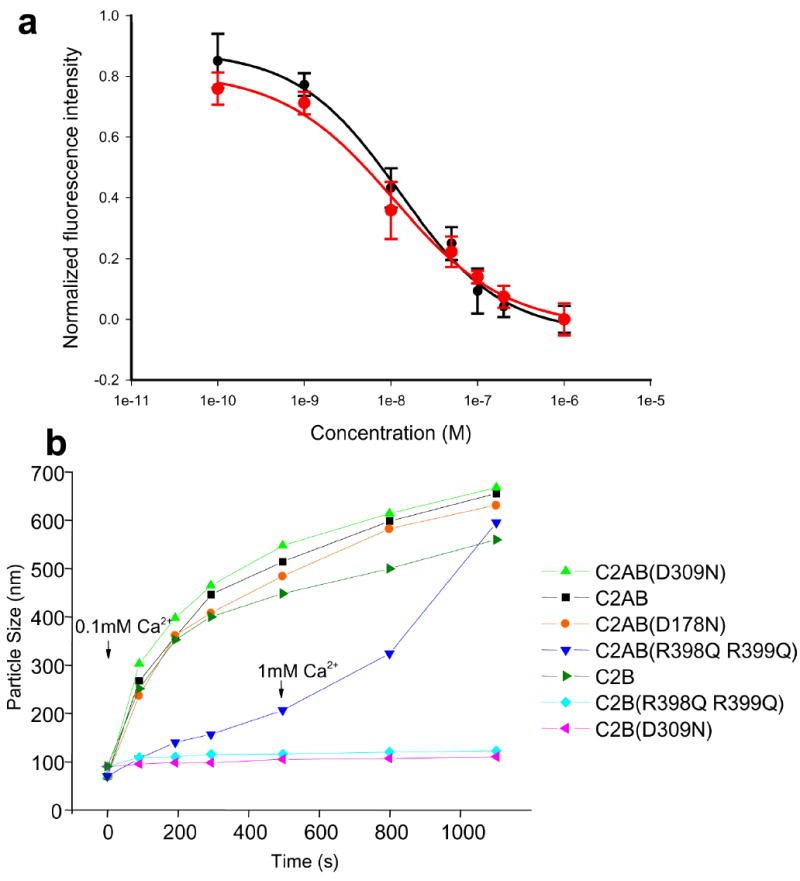
The R398Q,R399Q mutation impairs synaptotagmin-1/membrane interactions. (a) The R398Q,R399Q mutation does not impair the ability of the C2AB fragment to displace a complexin-1 fragment from membrane-anchored SNARE complexes. Supported phospholipid bilayers containing reconstituted SNARE complexes were deposited into microchannels, a rat complexin-1 fragment (residues 26-83) labeled with BODIPY-FL was bound to the SNARE complexes, and displacement of the labeled complexin 1 fragment by increasing concentrations of C2AB fragment (black circles) or C2AB-R398Q,R399Q fragment (red circles) was monitored with a confocal fluorescence microscope as described17, 30. The data were normalized to the background fluorescence. Error bars represent SEMs derived from three separate measurements. Fitting of the data to a dose-response curved yielded an EC50 of 12 nM for both the WT and mutant C2AB fragment, which is comparable to earlier results obtained for WT C2AB fragment30. (b) The R398Q,R399Q mutation impairs the vesicle clustering activity of the C2AB fragment. Mixtures of liposomes (100 nm average size) with WT or mutant C2B domain or C2AB fragment were prepared as described27, and the change in particle size as a function of time was measured by DLS after addition of 100 μM Ca2+. For the experiment performed with the R398Q,R399Q mutant C2AB fragment, 1 mM Ca2+ was added after 500 s.
