Abstract
Background
Autosomal dominant, nonsyndromic, midfrequency sensorineural hearing loss (SNHL) is a well-known clinical entity. There are no reported histopathologic studies of temporal bones from individuals with such a hearing loss.
Objectives
To describe the otopathology in 2 affected individuals from 2 different kindreds with nonsyndromic, dominant, midfrequency SNHL.
Material and Methods
Both subjects belonged to multigenerational families with nonsyndromic, autosomal dominant SNHL showing a cookie-bite pattern. Temporal bones were removed at autopsy and studied by light microscopy. Cytocochleograms were constructed for hair cells, stria vascularis, and cochlear neuronal cells.
Results
Subject 1 (a 77-yr-old man) from Kindred A was diagnosed in early childhood with an SNHL that was progressive, reaching profound levels by adulthood. Both cochleae showed complete loss of inner and outer hair cells, moderate to severe diffuse atrophy of the stria vascularis, and severe loss of cochlear neurons, including the peripheral dendrites. The hearing loss in Subject 2 (an 82-yr-old man from Kindred B) began in late childhood, was slowly progressive, and involved the higher frequencies later in life. Histopathology showed loss of outer and inner hair cells in the basal turn of the cochlea, moderate to severe loss of stria vascularis, but relative preservation of peripheral dendrites and cochlear neurons.
Conclusion
The main histopathologic abnormalities were loss of hair cells, stria vascularis, and cochlear neurons in 1 case and loss of hair cells and stria vascularis in the second case. Our results are consistent with the hypothesis that dysfunction and loss of hair cells may have been the primary histopathologic correlate for the midfrequency hearing losses in these 2 subjects.
Keywords: Sensorineural hearing loss, Temporal bone histopathology, Genetic deafness
Nonsyndromic, autosomal dominant, sensorineural hearing loss (SNHL) involving the middle frequencies, characterized by a cookie-bite, basin-shaped, or trough-shaped audiometric pattern, is a well-known clinical entity (1–7). A review of published reports describing this condition (1–7) indicates that the onset and progression of hearing loss varies between families. In many individuals, the hearing loss involves the higher frequencies later in life, which has been attributed to presbycusis, rather than the underlying genetic defect. In some cases, audiometric evaluation such as speech discrimination scores, short-increment sensitivity index, and tone decay testing has suggested a cochlear lesion as being responsible for the hearing loss. When tested, vestibular function has been reported to be normal (4).
Our review of the English language literature revealed a single citation regarding the temporal bone findings in this type of SNHL. Konigsmark et al. (4) cited a report by Paparella et al. (3) as describing the otopathology in genetically determined midfrequency SNHL. However, our review of the article by Paparella et al. (3) suggests otherwise. In the latter report, Paparella et al. described the otopathology in a 52-year-old man who had a bilateral, severe, flat, 60- to 70-dB SNHL. This individual’s daughter, aged 23 years, had audiometric documentation of a bilaterally symmetric, low-frequency SNHL (not a midfrequency loss). Therefore, it seems that the temporal bone pathologic finding in dominant midfrequency SNHL has not been previously described.
We have had the opportunity to examine temporal bones from 2 individuals belonging to 2 different kindreds with well-documented autosomal dominant, non-syndromic, midfrequency SNHL.
MATERIAL AND METHODS
Temporal bones were removed at autopsy and processed for light microscopy using fixation with 10% neutral-buffered formalin, decalcification using ethylenediaminetetraacetate, embedment in celloidin (3 ears) or polyester wax (1 ear), serial sectioning in the axial plane at a thickness of 20 μm for celloidin and 10 μm for polyester wax, and hematoxylin and eosin staining of every 10th section (7,8). All stained sections were examined by light microscopy. Graphic reconstruction of the cochlea was performed according to the method described by Schuknecht (7) to analyze loss of various neurosensory elements such as hair cells, stria vascularis, and cochlear neuronal cells. Cochlear neuronal cell counts were compared with normative age-matched data reported by Schuknecht (7).
RESULTS
Kindred A
The pedigree consists of individuals in 3 generations (Fig. 1A). Affected individuals demonstrate a hearing loss that is detected in early childhood. Audiometric evaluation at ages 2 to 5 years shows a midfrequency SNHL with a mild-moderate-mild pattern. The hearing loss is progressive, and some members of the family have received a cochlear implant. Affected individuals do not suffer from vestibular problems. A geneticist conducted an evaluation and concluded that this was a non-syndromic, autosomal dominant, midfrequency SNHL.
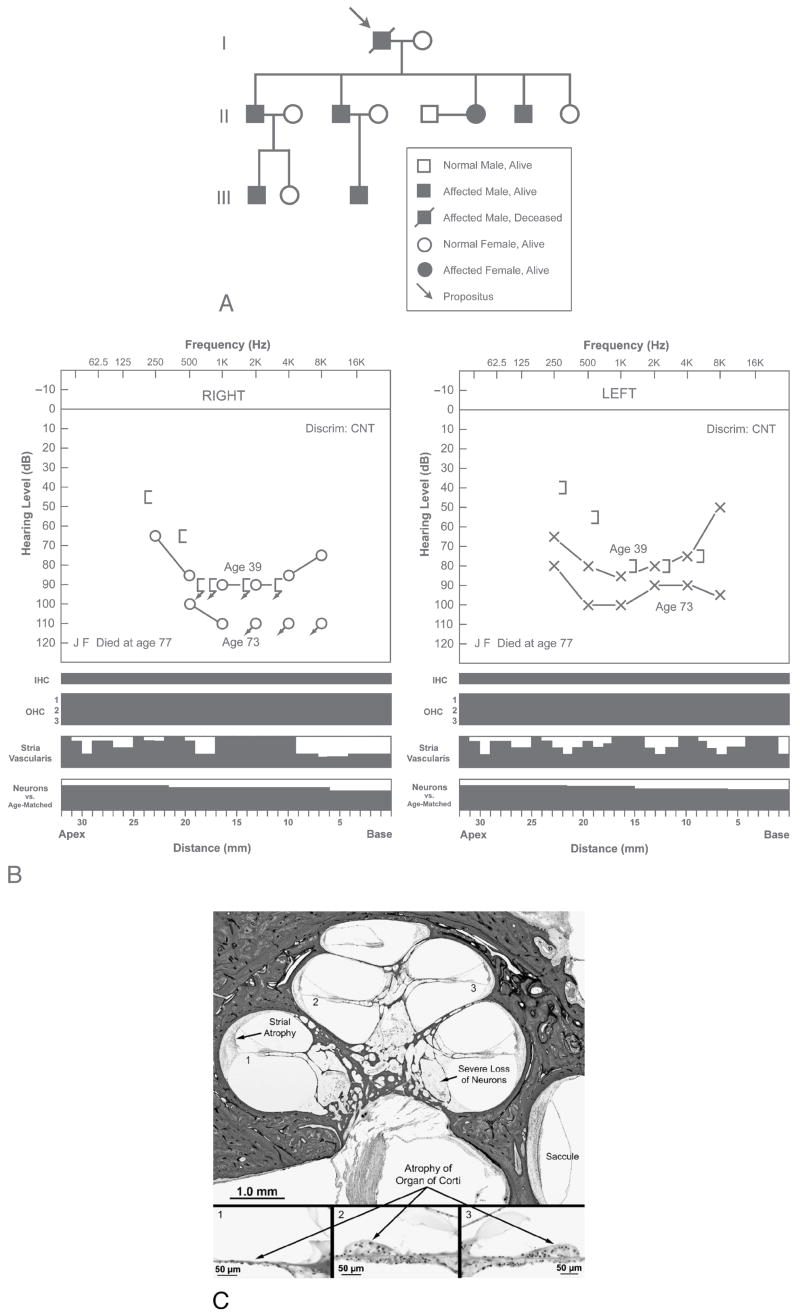
FIG. 1.
Subject 1 from Kindred A with autosomal dominant, nonsyndromic, midfrequency SNHL. A, Pedigree showing affected and nonaffected individuals in 3 generations. Affected individuals develop a midfrequency hearing loss in early childhood with mild-moderate-mild pattern on audiometric testing. They do not complain of vertigo. The propositus (Subject 1) was a 77-year-old man whose hearing loss was detected in early childhood. The hearing loss was progressive and reached severe to profound levels by the age of 50 years. B, Audio cytocochleograms of right and left ears of Subject 1. Audiometric thresholds at ages 39 and 73 years are shown. Speech discrimination could not be tested (CNT). The black areas in the cytocochleograms represent missing or abnormal elements. Inner hair (IHC) and outer hair cells (OHC) are shown as present (white) or absent (black). Vertical axes of the cytocochleogram for the stria vascularis and cochlear neurons represent percentage of loss. Neuronal counts were compared with age-matched controls. Both ears showed complete loss of inner and outer hair cells, severe atrophy of the stria vascularis, and severe loss of cochlear neurons. C, Histopathology of right cochlea of Subject 1. There was diffuse atrophy of the organ of Corti, severe atrophy of the stria vascularis, and severe loss of cochlear neurons.
The propositus (Subject 1) was diagnosed with hearing loss in early childhood. He was enrolled in a school for the deaf and hard of hearing and wore amplification in early life. The hearing loss was progressive, and audiometric evaluation at the age of 39 years showed a bilateral, nearly symmetric, severe SNHL, with the greatest loss in the middle frequencies (Fig. 1B). Speech discrimination tested at 95 dB sound pressure level was 12% on the right and 24% on the left. Similar audiometric results were obtained at ages 40, 43, 46, 49, and 51 years. Audiometry at age 73 years showed a severe to profound loss bilaterally: air conduction thresholds on the right were at 100 to 110 dB in the lower frequencies with no response for higher frequencies, whereas thresholds on the left were in the 80- to 100-dB range. He died at the age of 77 from complications of Legionnaire disease. The body was embalmed 12 hours after death, and both temporal bones were removed 35 hours postmortem.
Both temporal bones were nearly identical in appearance and are described together (Figs. 1B, C). The inner ear was fully developed. The cochlea showed 2.5 bony turns. There was a striking abnormality involving the organ of Corti, which was completely missing in the basal turn, and represented by supporting cells only in the remainder of the cochlea. Both inner and outer hair cells were missing throughout the cochlea. There was moderate to severe atrophy of the stria vascularis in all turns. There was near-complete loss of dendrites of cochlear neurons within the osseous spiral lamina. There was a severe loss of cochlear neurons within Rosenthal canal. The neuronal count was 6,165 on the right and 6,894 on the left, representing a 71 and 67% loss, respectively, when compared with age-matched controls. The central axons of the cochlear nerve showed atrophy, but the atrophy was less severe than that affecting the peripheral dendrites. The spiral ligament showed atrophy especially in the middle and apical turns, which was consistent with age-related change. The spiral limbus showed loss of fibrocytes and interdental cells in the basal turn (in areas where the organ of Corti was missing). The tectorial membrane seemed normal in all turns. All 5 vestibular sense organs showed good populations of hair cells, supporting cells, and neural innervation. There were small, inclusion-type cysts present in scattered locations in the cristae. The remainder of the temporal bone was unremarkable.
Kindred B
The propositus (Subject 2) belonged to the second generation of a 5-generation family with nonsyndromic, autosomal dominant, midfrequency SNHL (Fig. 2A). The family has been studied by author L.T. The hearing loss is postlingual in onset, and the midfrequency area is affected the most. Audiograms initially show a cookie-bite pattern. Later in life, affected individuals develop a more flat audiogram and hearing impairment in the higher frequencies.
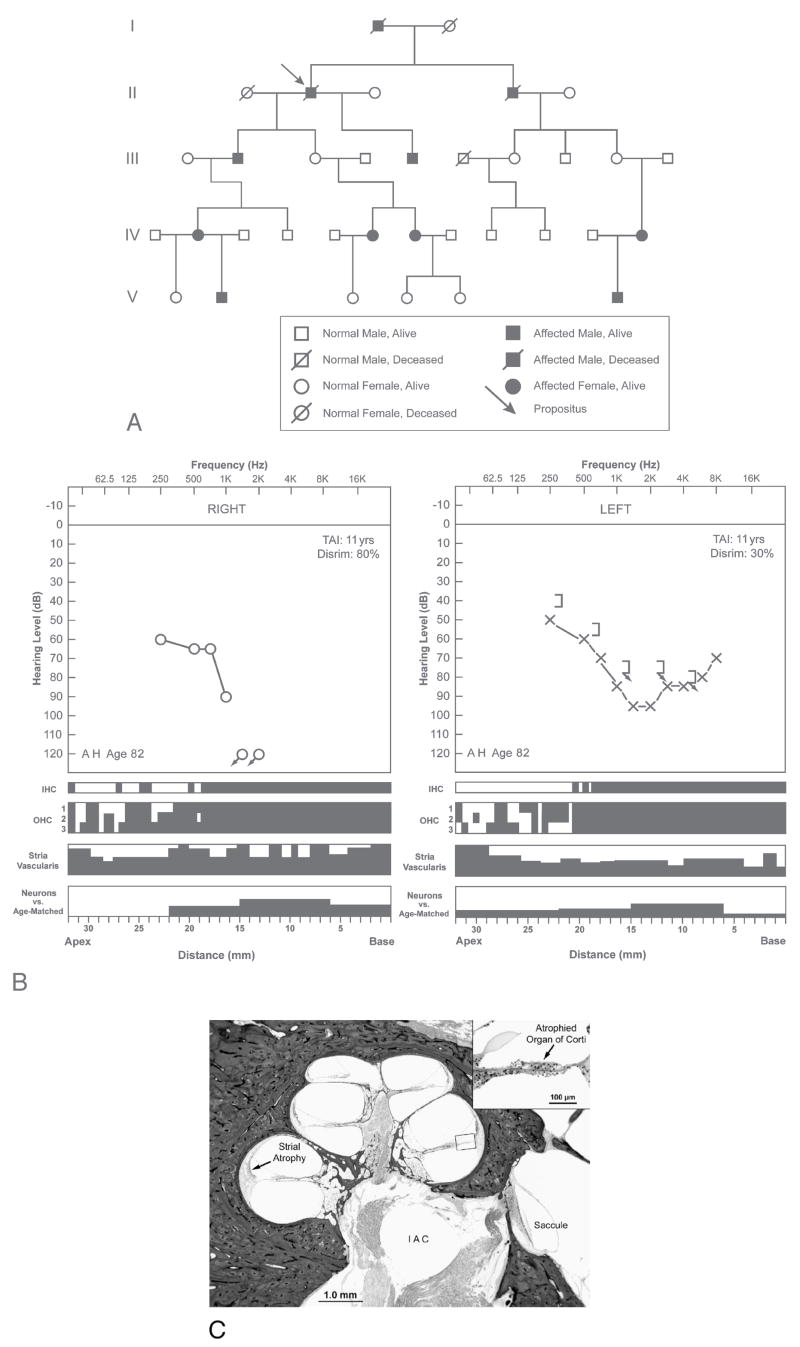
FIG. 2.
Subject 2 from Kindred B with autosomal dominant, nonsyndromic, midfrequency SNHL. A, Pedigree showing affected and nonaffected individuals in 5 generations. Affected individuals develop a midfrequency hearing loss in late childhood with slow progression. Later in life, the audiometric pattern becomes flat, with impaired hearing in the higher frequencies. The propositus (Subject 2) was an 82-year-old man who developed hearing loss in late childhood and wore amplification from approximately the age of 55 years. B, Audio cytocochleograms of right and left ears of Subject 2. Audiometric thresholds were analyzed at age 71 years, 11 years before death (test autopsy interval [TAI]). The right ear showed a severe to profound downsloping SNHL, whereas the left ear showed a moderate to severe midfrequency hearing loss. Speech discrimination was 80% on the right and 30% on the left. The cytocochleograms showed complete loss of outer and inner hair cells in the 0- to 19-mm region, with partial loss of outer hair cells in the remainder of the cochlea. There was moderate to severe atrophy of the stria vascularis. However, there was only a mild loss of cochlear neurons. C, Section from right cochlea of Subject 2. There was atrophy of the organ of Corti and moderate to severe atrophy of the stria vascularis. There was a relatively good population of cochlear neurons within Rosenthal canal (compare with Fig. 1C). The saccule was normal.
The propositus suffered from postlingual progressive SNHL. He wore amplification from approximately the age of 55 years. The hearing loss was gradually progressive, and audiometric evaluation at age 71 years showed the following (Fig. 2B). The right ear had a downsloping SNHL, demonstrating thresholds at 60 to 65 dB in the lower frequencies, falling to 90 dB at 1,000 Hz, with no response for higher frequencies. On the left, thresholds were 50 to 60 dB in the lower frequencies, falling to 95 dB in the midfrequencies, and then improving to 70 dB at 8,000 Hz. Speech discrimination was 80% on the right and 30% on the left. The patient died at the age of 82 years from small cell lung carcinoma. The body was kept refrigerated until removal of the temporal bones 90 hours after death. The right ear was embedded in celloidin and left in polyester wax.
The histopathologic findings were similar on the 2 sides and are described together (Figs. 2B, C). The inner ear and labyrinth were fully developed. The cochlea consisted of 2.5 bony turns. The organ of Corti was completely missing in the lower basal turn, represented by a mound of cells in the upper basal turn, and showed a more normal structure in the middle and apical turns. In the middle and apical turns, there was partial loss of outer hair cells, whereas inner hair cells were generally present. The stria vascularis showed moderate to severe atrophy in all turns. There was a mild loss of cochlear neurons compared with age-matched controls. The total cochlear neuronal count was 12,411 on the right (25% loss versus age-matched count) and 13,075 on the left (21% loss versus age-matched count). Peripheral dendrites were atrophic in the basal turn where the organ of Corti was missing, but dendrites were present in the middle and apical turns within the osseous spiral lamina. The spiral ligament showed atrophy in the middle and apical turns, consistent with age-related change. The spiral limbus showed mild atrophy in the proximal basal turn only. The tectorial membrane was detached from its attachment to the spiral limbus in the proximal basal turn. Both the limbus and the tectorial membrane were normal in the remainder of the cochlea. All 5 vestibular sense organs showed good populations of hair cells, supporting cells, and innervating dendrites. The remainder of the labyrinth and temporal bone was unremarkable.
DISCUSSION
We examined the otopathology in 2 subjects from 2 different kindreds with well-documented, nonsyndromic, autosomal dominant, midfrequency SNHL. In both kindreds, the hearing loss was noted in childhood and was progressive, eventually reaching severe to profound levels. The rate of progression of the hearing loss was greater in Subject 1.
Major otopathologic findings included hair cells loss, atrophy of the stria vascularis, and cochlear neuronal loss in Subject 1, and hair cell loss with atrophy of the stria vascularis in Subject 2. A notable difference between the 2 individuals was the significantly greater loss of cochlear neurons and peripheral dendrites in Subject 1. The difference in rate of progression of the hearing loss and in the histopathologic findings between these 2 individuals is indicative of phenotypic variability in this type of SNHL.
In general, in both individuals, the degree of otopathology was greater than what might be expected from the audiometric thresholds. For example, in Subject 1, there was complete loss of inner and outer hair cells throughout the cochlea, which should manifest as anacusis. Similarly, no inner or outer hair cells were present in both ears of Subject 2 in the basal 19 mm, which should correspond to profound losses for frequencies 1,000 Hz and higher. One explanation for the discrepancy between the audiometric and histopathologic data is that the last audiogram was done several years before death in both cases, and it is possible that the hearing loss progressed in the time interval between the last audiogram and death.
The severe otopathology (atrophy of hair cells, stria, and cochlear neurons) in both cases probably represents end-stage changes in these individuals. The findings do not allow one to analyze with certainty the precise histopathologic correlate for the midfrequency pattern of the hearing loss. Strial atrophy is generally associated with a flat SNHL, and cochlear neuronal loss degrades speech discrimination before causing threshold shifts (7). Hence, the obvious candidate to explain the midfrequency dip is loss of hair cells. In principle, dysfunction and loss of hair cells in the middle regions of the cochlea can result in a midfrequency threshold shift. Because the hair cell dysfunction spreads apically and basally, lower and higher frequencies would be affected. Testing this hypothesis will require examination of temporal bones from individuals whose hearing losses have not reached profound levels.
Genetic studies have revealed that dominant midfrequency hearing loss can be the result of mutations of at least 4 different loci, with the gene identified for 3 of these: DFNA8/12 (α-tectorin gene; [9–11]), DFNA10 (EYA4 gene; [12,13]), DFNA13 (COL11A2 gene; [5,14,15]), and DFNA21 (16). There was insufficient power in the kindreds to perform linkage studies, and appropriate tissue (such as blood) was not available from our 2 cases to sequence the 3 genes known to cause dominant midfrequency loss. Thus, the genetic defect responsible for the hearing loss in our 2 kindreds is not known.
SUMMARY AND CONCLUSION
We examined the otopathology in 2 individuals from 2 different kindreds with nonsyndromic, autosomal dominant, midfrequency SNHL. In both subjects, the hearing loss was detected in childhood and was progressive, affecting other frequencies with the passage of time. The main histopathologic abnormalities were loss of hair cells, stria vascularis, and cochlear neurons in 1 case and loss of hair cells and stria vascularis in the second case. Vestibular sense organs were not affected in either case. Our studies are consistent with the hypothesis that dysfunction and loss of hair cells may have been the primary histopathologic correlate for the midfrequency hearing losses in these 2 subjects.
Acknowledgments
The authors thank Axel Eliasen and Lakshmi Mittal for support of our work.
This study was supported by Grant U24DC0008559 from the National Institute on Deafness and Other Communication Disorders.
References
- 1.Mårtensson B. Dominant hereditary nerve deafness. Acta Otolaryngol (Stockh) 1960;52:270–4. doi: 10.3109/00016486009123147. [DOI] [PubMed] [Google Scholar]
- 2.Williams F, Roblee LA. Hereditary nerve deafness. A follow-up of four cases in one family. Arch Otolaryngol. 1962;75:69–77. doi: 10.1001/archotol.1962.00740040073007. [DOI] [PubMed] [Google Scholar]
- 3.Paparella MM, Sugiura S, Hoshino T. Familial progressive sensorineural deafness. Arch Otolaryngol. 1969;90:44–51. doi: 10.1001/archotol.1969.00770030046010. [DOI] [PubMed] [Google Scholar]
- 4.Konigsmark BW, Salman S, Haskins H, Mengel M. Dominant midfrequency hearing loss. Ann Otol Rhinol Laryngol. 1970;79:42–53. doi: 10.1177/000348947007900105. [DOI] [PubMed] [Google Scholar]
- 5.Kunst H, Huybrechts C, Marres H, Huygen P, Van Camp G, Cremers C. The phenotype of DFNA13/COL11A2: nonsyndromic autosomal dominant mid-frequency and high-frequency sensorineural hearing impairment. Am J Otol. 2000;2:181–7. doi: 10.1016/s0196-0709(00)80006-x. [DOI] [PubMed] [Google Scholar]
- 6.Kaksonen R, Widen E, Cormand B, et al. Autosomal dominant midfrequency hearing impairment. Scand Audiol Suppl. 2001;52:85–7. doi: 10.1080/010503901300007164. [DOI] [PubMed] [Google Scholar]
- 7.Schuknecht HF. Ear. 2. Philadelphia, PA: Lea and Febiger; 1993. [Google Scholar]
- 8.Merchant SN, Burgess B, O’Malley J, Jones D, Adams JC. Polyester wax: a new embedding medium for the histopathologic study of human temporal bones. Laryngoscope. 2006;116:245–9. doi: 10.1097/01.mlg.0000192171.85406.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Govaerts PJ, De Ceulaer G, Daemers K, et al. A new autosomal-dominant locus (DFNA12) is responsible for a nonsyndromic, mid-frequency, prelingual and nonprogressive sensorineural hearing loss. Am J Otol. 1998;6:718–23. [PubMed] [Google Scholar]
- 10.Verhoeven K, Van Laer L, Kirschhofer K, et al. Mutations in the human alpha-tectorin gene cause autosomal dominant non-syndromic hearing impairment. Nat Genet. 1998;1:60–2. doi: 10.1038/ng0598-60. Erratum in: Nat Genet 1999;4;449. [DOI] [PubMed] [Google Scholar]
- 11.Pfister M, Thiele H, Van Camp G, et al. A genotype-phenotype correlation with gender-effect for hearing impairment caused by TECTA mutations. Cell Physiol Biochem. 2004;14:369–76. doi: 10.1159/000080347. [DOI] [PubMed] [Google Scholar]
- 12.De Leenheer EM, Huygen PL, Wayne S, Smith RJ, Cremers CW. The DFNA10 phenotype. Ann Otol Rhinol Laryngol. 2001;9:861–6. doi: 10.1177/000348940111000910. [DOI] [PubMed] [Google Scholar]
- 13.Wayne S, Robertson NG, DeClau F, et al. Mutations in the transcriptional activator EYA4 cause late-onset deafness at the DFNA10 locus. Hum Mol Genet. 2001;3:195–200. doi: 10.1093/hmg/10.3.195. [DOI] [PubMed] [Google Scholar]
- 14.McGuirt WT, Prasad SD, Griffith AJ, et al. Mutations in COL11A2 cause non-syndromic hearing loss (DFNA13) Nat Genet. 1999;4:413–9. doi: 10.1038/70516. [DOI] [PubMed] [Google Scholar]
- 15.De Leenheer EM, Kunst H, McGuirt WT, et al. Autosomal dominant inherited hearing impairment caused by a missense mutation in COL11A2 (DFNA13) Arch Otolaryngol Head Neck Surg. 2001;127:13–7. doi: 10.1001/archotol.127.1.13. [DOI] [PubMed] [Google Scholar]
- 16.Kunst H, Marres H, Huygen P, et al. Non-syndromic autosomal dominant progressive non-specific mid-frequency sensorineural hearing impairment with childhood to late adolescence onset (DFNA21) Clin Otolaryngol Allied Sci. 25:45–54. doi: 10.1046/j.1365-2273.2000.00327.x. [DOI] [PubMed] [Google Scholar]