Abstract
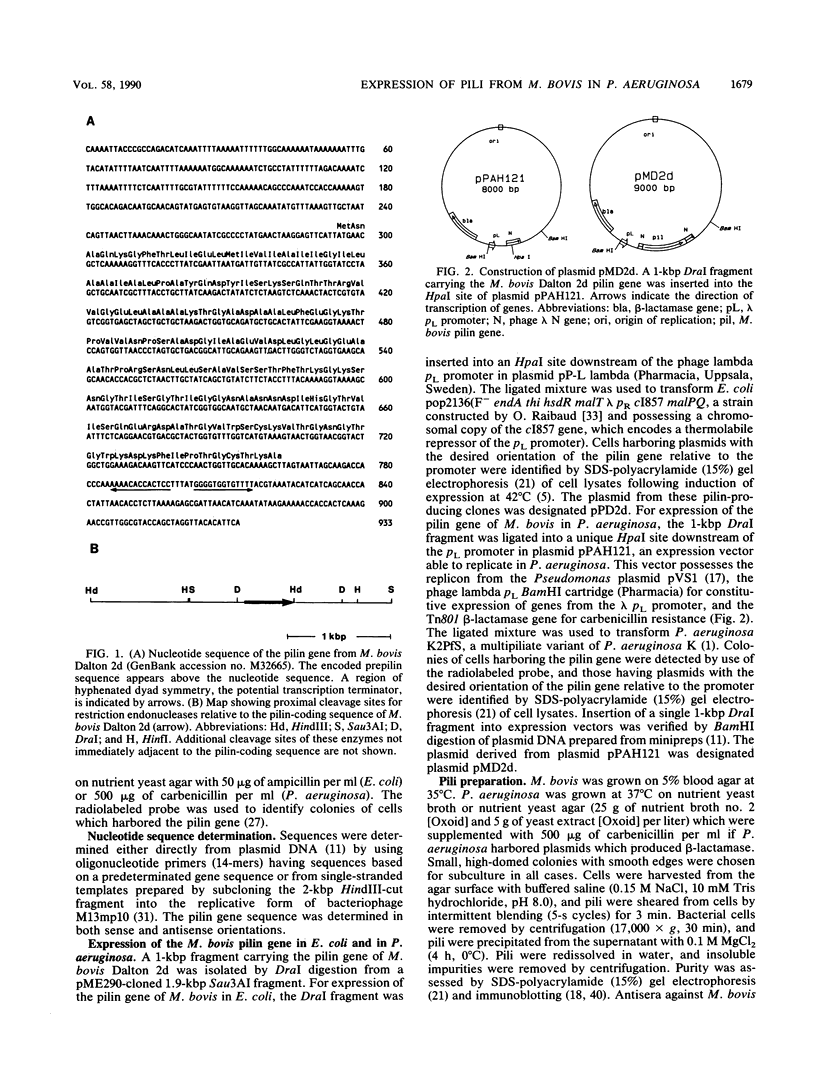
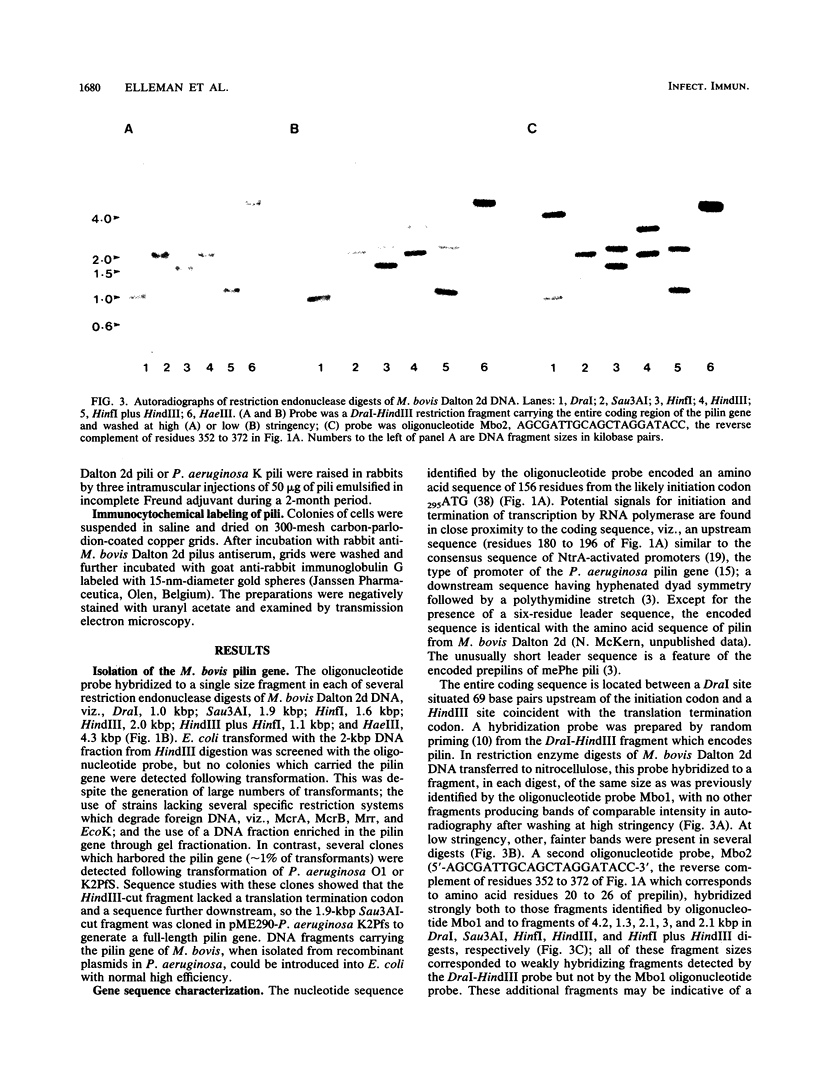
The pilin gene of Moraxella bovis Dalton 2d was isolated by cloning in Pseudomonas aeruginosa. The nucleotide sequence of this gene encodes a prepilin of 156 amino acid residues. When high levels of pilin were expressed from the gene in P. aeruginosa, by using the pL promoter of bacteriophage lambda inserted upstream of the coding sequence, pili which were indistinguishable from pili of M. bovis were produced.
Full text
PDF
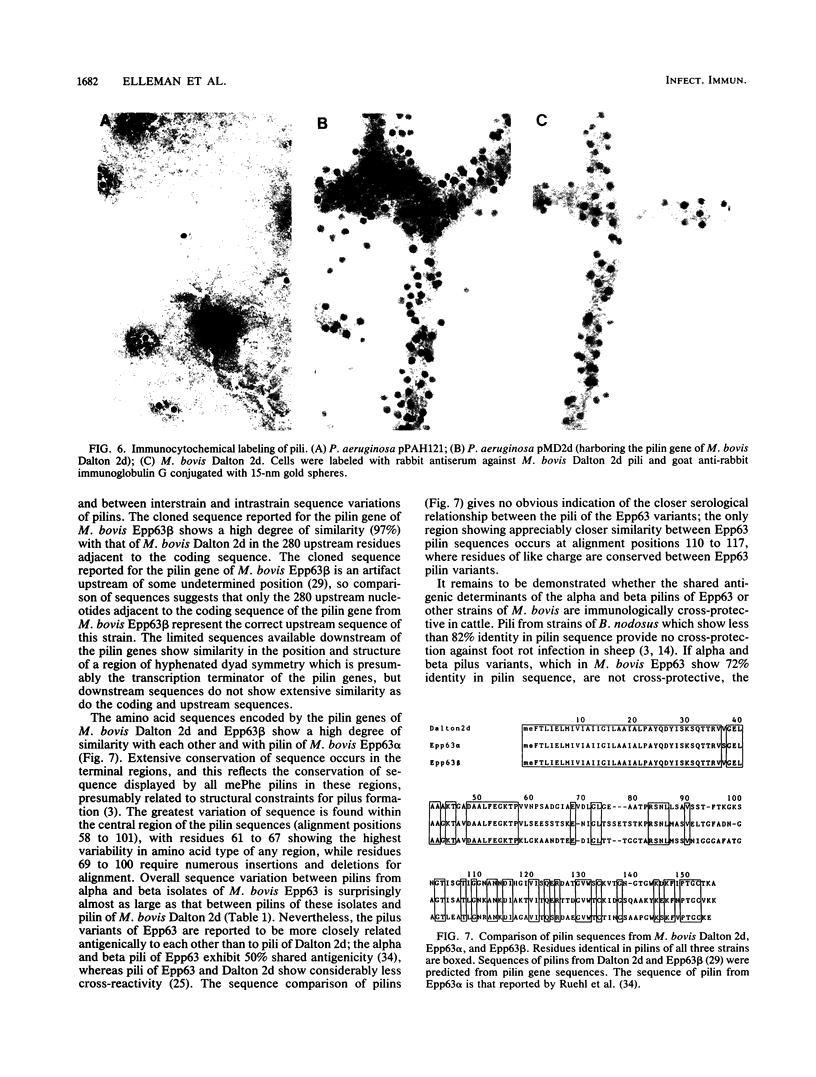

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradley D. E. The adsorption of Pseudomonas aeruginosa pilus-dependent bacteriophages to a host mutant with nonretractile pili. Virology. 1974 Mar;58(1):149–163. doi: 10.1016/0042-6822(74)90150-0. [DOI] [PubMed] [Google Scholar]
- Elleman T. C., Hoyne P. A., Emery D. L., Stewart D. J., Clark B. L. Expression of the pilin gene from Bacteroides nodosus in Escherichia coli. Infect Immun. 1986 Jan;51(1):187–192. doi: 10.1128/iai.51.1.187-192.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elleman T. C., Hoyne P. A., Emery D. L., Stewart D. J., Clark B. L. Isolation of the gene encoding pilin of Bacteroides nodosus (strain 198), the causal organism of ovine footrot. FEBS Lett. 1984 Jul 23;173(1):103–107. doi: 10.1016/0014-5793(84)81026-1. [DOI] [PubMed] [Google Scholar]
- Elleman T. C., Hoyne P. A., McKern N. M., Stewart D. J. Nucleotide sequence of the gene encoding the two-subunit pilin of Bacteroides nodosus 265. J Bacteriol. 1986 Jul;167(1):243–250. doi: 10.1128/jb.167.1.243-250.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elleman T. C., Hoyne P. A., Stewart D. J., McKern N. M., Peterson J. E. Expression of pili from Bacteroides nodosus in Pseudomonas aeruginosa. J Bacteriol. 1986 Nov;168(2):574–580. doi: 10.1128/jb.168.2.574-580.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elleman T. C. Pilins of Bacteroides nodosus: molecular basis of serotypic variation and relationships to other bacterial pilins. Microbiol Rev. 1988 Jun;52(2):233–247. doi: 10.1128/mr.52.2.233-247.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elleman T. C., Stewart D. J. Efficacy against footrot of a Bacteroides nodosus 265 (serogroup H) pilus vaccine expressed in Pseudomonas aeruginosa. Infect Immun. 1988 Mar;56(3):595–600. doi: 10.1128/iai.56.3.595-600.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Every D., Skerman T. M. Protection of sheep against experimental footrot by vaccination with pili purified from Bacteroides nodosus. N Z Vet J. 1982 Oct;30(10):156–158. doi: 10.1080/00480169.1982.34921. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Hattori M., Sakaki Y. Dideoxy sequencing method using denatured plasmid templates. Anal Biochem. 1986 Feb 1;152(2):232–238. doi: 10.1016/0003-2697(86)90403-3. [DOI] [PubMed] [Google Scholar]
- Hawkes R., Niday E., Gordon J. A dot-immunobinding assay for monoclonal and other antibodies. Anal Biochem. 1982 Jan 1;119(1):142–147. doi: 10.1016/0003-2697(82)90677-7. [DOI] [PubMed] [Google Scholar]
- Holloway B. W. Genetics of Pseudomonas. Bacteriol Rev. 1969 Sep;33(3):419–443. doi: 10.1128/br.33.3.419-443.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyne P. A., Elleman T. C., McKern N. M., Stewart D. J. Sequence of pilin from Bacteroides nodosus 351 (Serogroup H) and implications for serogroup classification. J Gen Microbiol. 1989 May;135(5):1113–1122. doi: 10.1099/00221287-135-5-1113. [DOI] [PubMed] [Google Scholar]
- Ishimoto K. S., Lory S. Formation of pilin in Pseudomonas aeruginosa requires the alternative sigma factor (RpoN) of RNA polymerase. Proc Natl Acad Sci U S A. 1989 Mar;86(6):1954–1957. doi: 10.1073/pnas.86.6.1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh Y., Haas D. Cloning vectors derived from the Pseudomonas plasmid pVS1. Gene. 1985;36(1-2):27–36. doi: 10.1016/0378-1119(85)90066-6. [DOI] [PubMed] [Google Scholar]
- Itoh Y., Watson J. M., Haas D., Leisinger T. Genetic and molecular characterization of the Pseudomonas plasmid pVS1. Plasmid. 1984 May;11(3):206–220. doi: 10.1016/0147-619x(84)90027-1. [DOI] [PubMed] [Google Scholar]
- Johnson K., Parker M. L., Lory S. Nucleotide sequence and transcriptional initiation site of two Pseudomonas aeruginosa pilin genes. J Biol Chem. 1986 Nov 25;261(33):15703–15708. [PubMed] [Google Scholar]
- Klemm P. Fimbrial adhesions of Escherichia coli. Rev Infect Dis. 1985 May-Jun;7(3):321–340. doi: 10.1093/clinids/7.3.321. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lederberg E. M., Cohen S. N. Transformation of Salmonella typhimurium by plasmid deoxyribonucleic acid. J Bacteriol. 1974 Sep;119(3):1072–1074. doi: 10.1128/jb.119.3.1072-1074.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehr C., Jayappa H. G., Goodnow R. A. Serologic and protective characterization of Moraxella bovis pili. Cornell Vet. 1985 Oct;75(4):484–492. [PubMed] [Google Scholar]
- Lepper A. W., Power B. E. Infectivity and virulence of Australian strains of Moraxella bovis for the murine and bovine eye in relation to pilus serogroup sub-unit size and degree of piliation. Aust Vet J. 1988 Oct;65(10):305–309. doi: 10.1111/j.1751-0813.1988.tb14512.x. [DOI] [PubMed] [Google Scholar]
- Lepper A. W. Vaccination against infectious bovine keratoconjunctivitis: protective efficacy and antibody response induced by pili of homologous and heterologous strains of Moraxella bovis. Aust Vet J. 1988 Oct;65(10):310–316. doi: 10.1111/j.1751-0813.1988.tb14513.x. [DOI] [PubMed] [Google Scholar]
- Marrs C. F., Ruehl W. W., Schoolnik G. K., Falkow S. Pilin-gene phase variation of Moraxella bovis is caused by an inversion of the pilin genes. J Bacteriol. 1988 Jul;170(7):3032–3039. doi: 10.1128/jb.170.7.3032-3039.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrs C. F., Schoolnik G., Koomey J. M., Hardy J., Rothbard J., Falkow S. Cloning and sequencing of a Moraxella bovis pilin gene. J Bacteriol. 1985 Jul;163(1):132–139. doi: 10.1128/jb.163.1.132-139.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattick J. S., Bills M. M., Anderson B. J., Dalrymple B., Mott M. R., Egerton J. R. Morphogenetic expression of Bacteroides nodosus fimbriae in Pseudomonas aeruginosa. J Bacteriol. 1987 Jan;169(1):33–41. doi: 10.1128/jb.169.1.33-41.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messing J. New M13 vectors for cloning. Methods Enzymol. 1983;101:20–78. doi: 10.1016/0076-6879(83)01005-8. [DOI] [PubMed] [Google Scholar]
- Pugh G. W., Jr, Hughes D. E., Booth G. D. Experimentally induced infections bovine keratoconjunctivitis: effectiveness of a pilus vaccine against exposure to homologous strains of Moraxella bovis. Am J Vet Res. 1977 Oct;38(10):1519–1522. [PubMed] [Google Scholar]
- Raibaud O., Mock M., Schwartz M. A technique for integrating any DNA fragment into the chromosome of Escherichia coli. Gene. 1984 Jul-Aug;29(1-2):231–241. doi: 10.1016/0378-1119(84)90183-5. [DOI] [PubMed] [Google Scholar]
- Ruehl W. W., Marrs C. F., Fernandez R., Falkow S., Schoolnik G. K. Purification, characterization, and pathogenicity of Moraxella bovis pili. J Exp Med. 1988 Sep 1;168(3):983–1002. doi: 10.1084/jem.168.3.983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith G. E., Summers M. D. The bidirectional transfer of DNA and RNA to nitrocellulose or diazobenzyloxymethyl-paper. Anal Biochem. 1980 Nov 15;109(1):123–129. doi: 10.1016/0003-2697(80)90019-6. [DOI] [PubMed] [Google Scholar]
- Stewart D. J., Elleman T. C. A Bacteroides nodosus pili vaccine produced by recombinant DNA for the prevention and treatment of foot-rot in sheep. Aust Vet J. 1987 Mar;64(3):79–81. doi: 10.1111/j.1751-0813.1987.tb09620.x. [DOI] [PubMed] [Google Scholar]
- Stewart D. J. The role of various antigenic fractions of Bacteroides nodosus in eliciting protection against foot-rot in vaccinated sheep. Res Vet Sci. 1978 Jan;24(1):14–19. [PubMed] [Google Scholar]
- Stormo G. D., Schneider T. D., Gold L. M. Characterization of translational initiation sites in E. coli. Nucleic Acids Res. 1982 May 11;10(9):2971–2996. doi: 10.1093/nar/10.9.2971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor R. K., Miller V. L., Furlong D. B., Mekalanos J. J. Use of phoA gene fusions to identify a pilus colonization factor coordinately regulated with cholera toxin. Proc Natl Acad Sci U S A. 1987 May;84(9):2833–2837. doi: 10.1073/pnas.84.9.2833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]