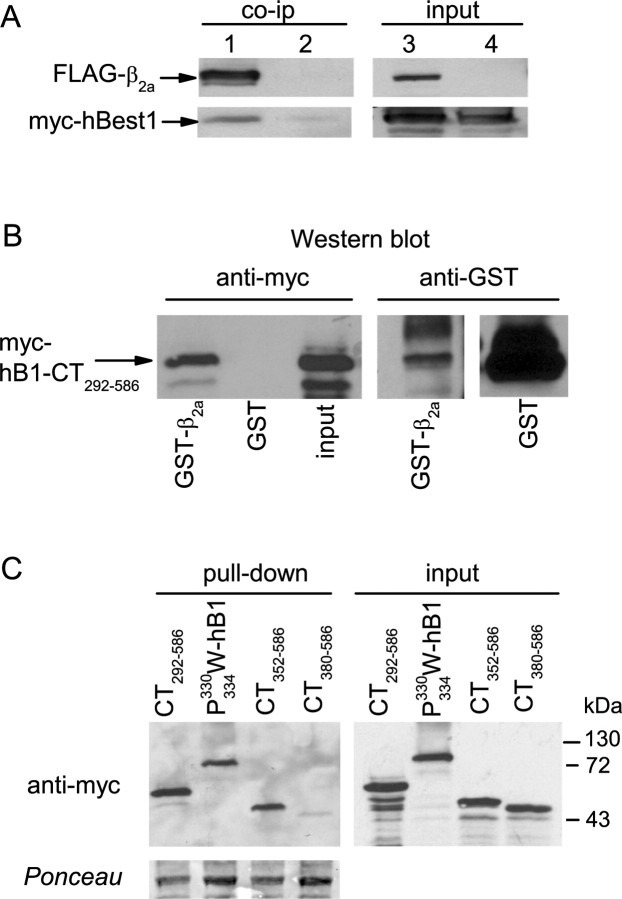
Figure 9.
Binding of hbest1 to CaVβ2a. A, Coimmunoprecipitation (co-ip) of full-length myc-hBest1 with FLAG-tagged β2a. myc-hBest1 was cotransfected with FLAG-β2a (lanes 1, 3) or untagged β2a (lanes 2, 4) as a negative control in HEK293 cells. Cell lysates were incubated with FLAG antibodies, and immunoprecipitated proteins (left) were detected by Western blot with anti-FLAG or myc antibodies. Input lanes (right) represent ∼5% of cell lysate used for the assay. B, Pull-down of myc-tagged hBest1 C terminus (CT292–586) from transfected HEK293 cell lysates by GST-β2a but not GST. Bound protein was detected by Western blot with anti-myc antibodies (left). The blot with anti-GST antibodies (right) shows levels of GST-proteins used in assay. C, Pull-down of myc-tagged hBest1 mutation and CT fragments by GST-β2a (left). Ponceau staining shows levels of GST-β2a used for pull-down. Input lanes (right) represent ∼5% of cell lysate used for the assay.