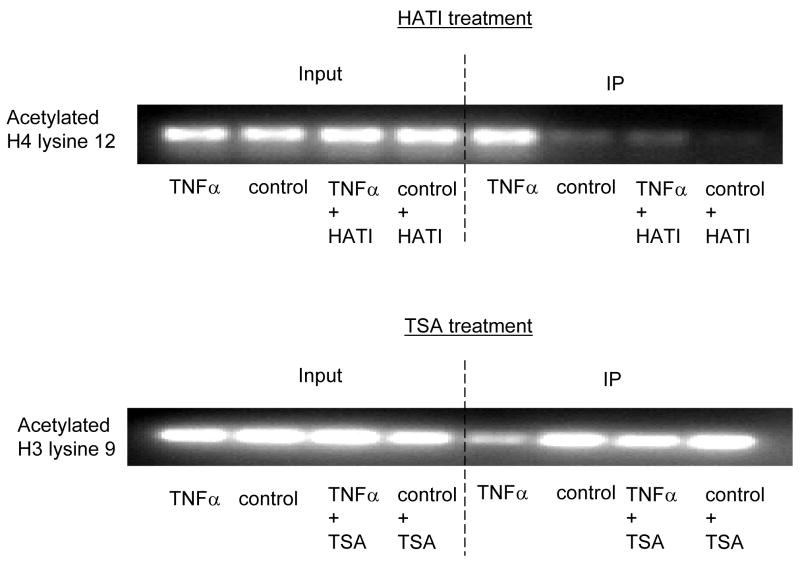
Fig. 4. TNFα-JNK1 induced modification.
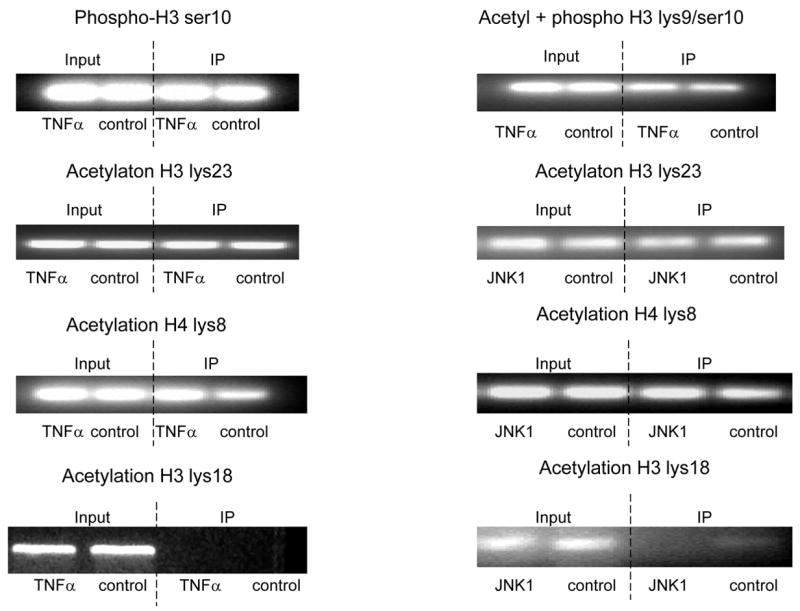
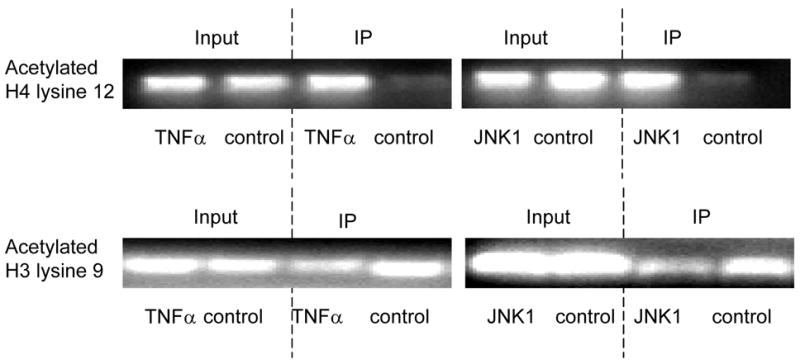
ChIP assays were performed with specific histone (H) antibodies in HAMSCs treated with or without TNFα or JNK1. Primers covering the −60 to +104bp region of the P4Hα1 promoter were used to amplify histone-bound DNA in PCR. A. ChIP assays using anti-phospho-H3 serine10, acetyl/phospho-H3 lysine9/serine10, acetyl-H3 lysine23, acetyl-H4 lysine8 and acetyl-H3 lysine18 antibodies. Experiments show no change in the acetylation or phosphorylation in H3 and H4 at the listed amino acid sites. B. ChIP assay using anti-acetyl-H4 lysine 12 or anti-acetyl-H3 lysine 9 in cells treated with or without TNFα or JNK1. TNFα or JNK1 treatment induced H4-lysine 12 acetylation and H3-lysine 9 deacetylation. C. HASMCs treated with or without TNFα were further treated with 20 μM HATI or 500ng/ml TSA (HDAC inhibitor). ChIP assays were performed using anti-acetyl-H4 lysine 12 or anti-acetyl-H3 lysine 9 antibodies. HATI treatment inhibited the TNFα-induced H4 lysine12 acetylation; TSA blocked the TNFα-induced deacetylation of H3 lysine. Exposure to HATI or TSA alone did not change the acetylation status at lysine 9 of H3 and lysine 12 of H4.