Table 1.
Scope of Ynamide-Kinugasa Reaction
| entry | ynamides | α-amino-β-lactams | yield [%]a | dr: [a:b]b | ||
|---|---|---|---|---|---|---|
| 1 | 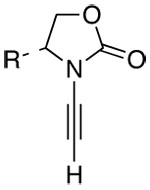 |
7 | 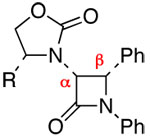 |
12: R = Ph | 80 | ≥95:5 |
| 2 | 8 | 13: R = i-Pr | 36 | ≥ 95:5 | ||
| 3 | 9 | 14: R = CHPh2 | 28c,d | nde | ||
| 4 | 7 | 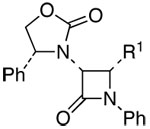 |
15: R1 = 4-Br-Ph | 77 | ≥95:5 | |
| 5 | 7 | 16: R1 = 1Naph | 71 | 90:10 | ||
| 6 | 7 | 17: R1 = styryl | 72c,f | 93:7 | ||
| 7 | 7 | 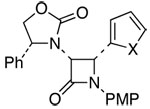 |
18: X = O | 61c | 82:18 | |
| 8 | 7 | 19: X = S | 60c,g | ≥95:5 | ||
| 9 | 7 | 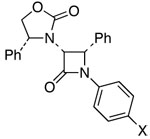 |
20: X = Cl | 65 | ≥95:5 | |
| 10 | 7 | 21: X = CO2Et | 63 | ≥95:5 | ||
| 11 | 5 | 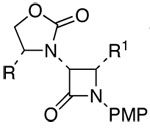 |
22: R = Bn; R1 = Ph | 59h | 91:9 | |
| 12 | 7 | 23: R = Ph; R1 = Ph | 60h | ≥95:5 | ||
| 13 | 7 | 24: R = Ph; R1 = c-hex | 60c,i,h | 92:8 | ||
| 14 | 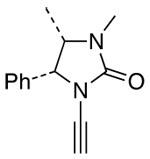 |
10 | 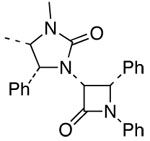 |
25 | 61 | ≥95:5 |
| 15 | 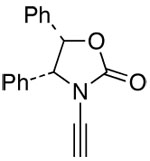 |
11 | 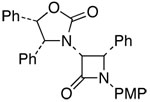 |
26 | 60c,h | ≥95:5 |
Reaction conditions are as shown in Scheme 2 unless otherwise indicated. All are isolated yields.
dr is determined using 1H NMR. All isomers-a are cis. The minor isomer-b is trans.
0.2 equiv of CuI was used, and the reaction was run at 0 °C to rt.
With 0.2 equiv of CuCl, the yield was 13%.
nd: Not determined.
With 0.2 equiv of CuCl, yield was 71% and dr = 91:9.
With 0.2 equiv of CuCl, yield was 48% and dr = 86:14.
PMP: para-methoxyphenyl.
With 0.2 equiv of CuCl and 4.8 equiv of Hünig's base, yield was 54% and dr = 87:13.
