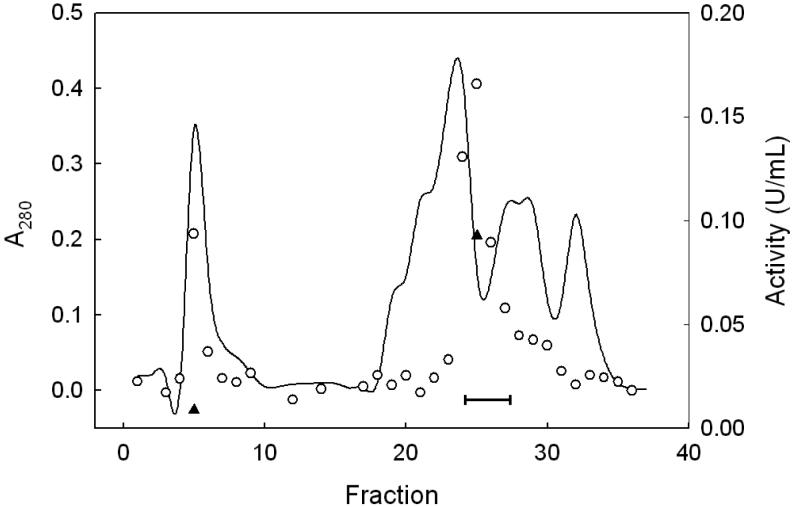
Figure 1.
DEAE-Sepharose column chromatography: protein concentration and peroxidase activity profiles. KME Buffer A was pumped through the column at 1.0 mL/min, with 10-mL fractions being collected. The linear elution gradient (0-100% KME Buffer B) was started at fraction 16 and continued to fraction 32, 100% Buffer B being used thereafter. Protein level was measured by absorbance at 280 nm (solid line). Enzymatic activity was determined by coupled spectrophotometric assay, using CuOOH (○) or PCOOH (▲) as the peroxide substrate. One unit (U) of activity corresponds to 1 μmol of CuOOH or PCOOH consumed per min. Bar indicates fractions that were pooled for further purification.