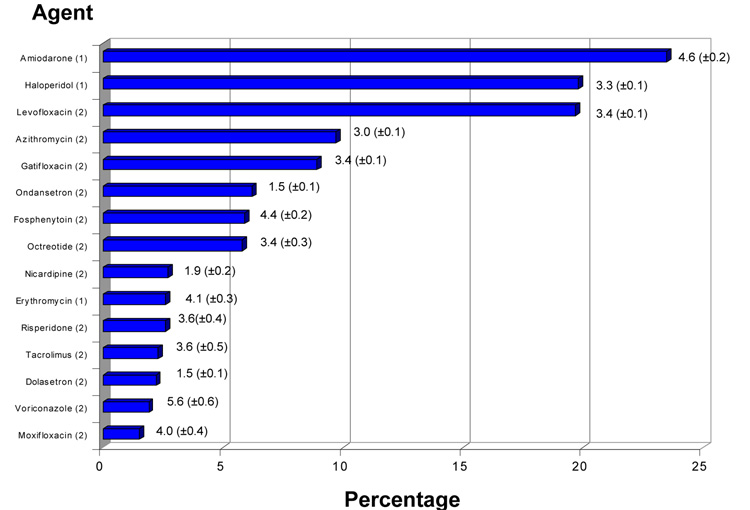
Figure 1. Administration of QT-interval prolonging drugs in critical illness.
Frequency of QT-interval prolonging drug administration (as percentage of all QT-interval prolonging drugs administered) with associated mean (±SE) duration (days). Analysis was limited to drugs comprising at least 1% of all QT-interval prolonging drugs prescribed. Numbers in parentheses adjacent to drug names refer to the categorization with respect to risk of drug associated arrhythmia. As noted in Table 1, a designation of ‘1’ denotes generally accepted to have a risk of causing torsades de pointes while ‘2’ denotes reported association with torsades de pointes but for which substantial evidence is lacking(4). As this figure illustrates, with the exception of amiodarone, haloperidol, and erythromycin, most drugs administered to critically ill patients carry a designation of ‘2’.