Figure 4.
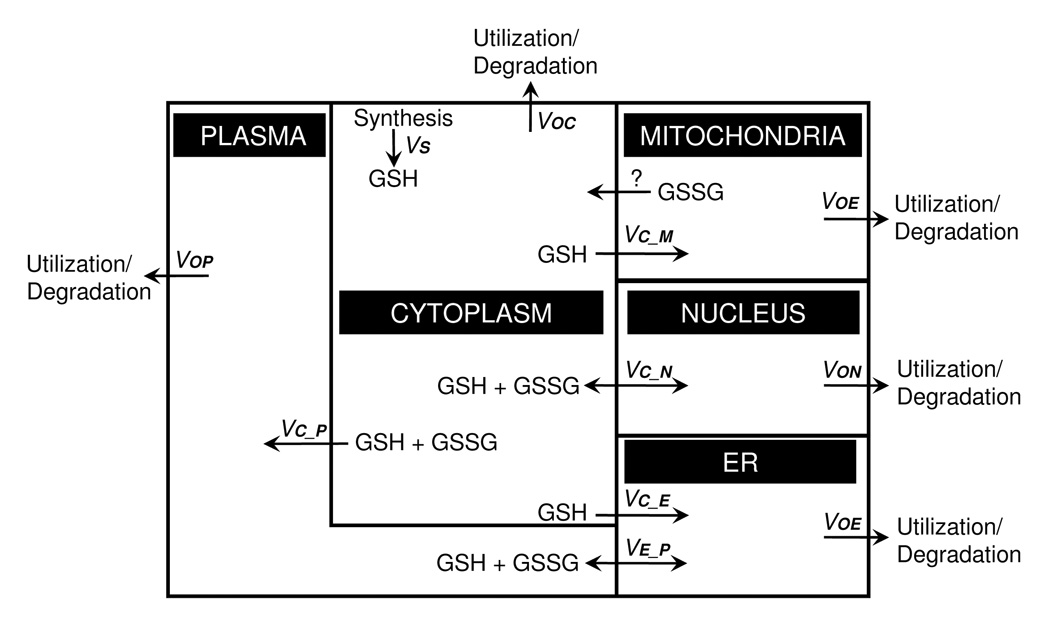
A compartmental model of steady-state glutathione fluxes. Under homeostatic conditions, GSH transport must be balanced by degradation/utilization to balance the rate of cytosolic GSH synthesis. In the diagram, GSH influx to the ER, mitochondria and plasma is considered to be unidirectional, while the exocytosis of metabolites through the ER will release GSH and GSSG into the plasma at the same rate. Mitochondrial loss of GSH is poorly understood but could occur by unidirectional efflux of GSSG. Passive transport to the nucleus will be bidirectional and include GSH + GSSG. Salient features revealed by this formulation include: 1) an uncharacterized mechanism for loss of GSH or GSSG from mitochondria is needed to balance mitochondrial GSH/GSSG redox state, 2) non-equal partitioning of reductases and oxidases between the cytoplasm and nucleus could maintain a disequilibrium between compartments, 3) differential regulation of GSH and GSSG transport between compartments can determine differences in steady-state redox potential between compartments, and 4) the secretory pathway could provide a kinetically important route for electron transfer between cytoplasm and plasma.
