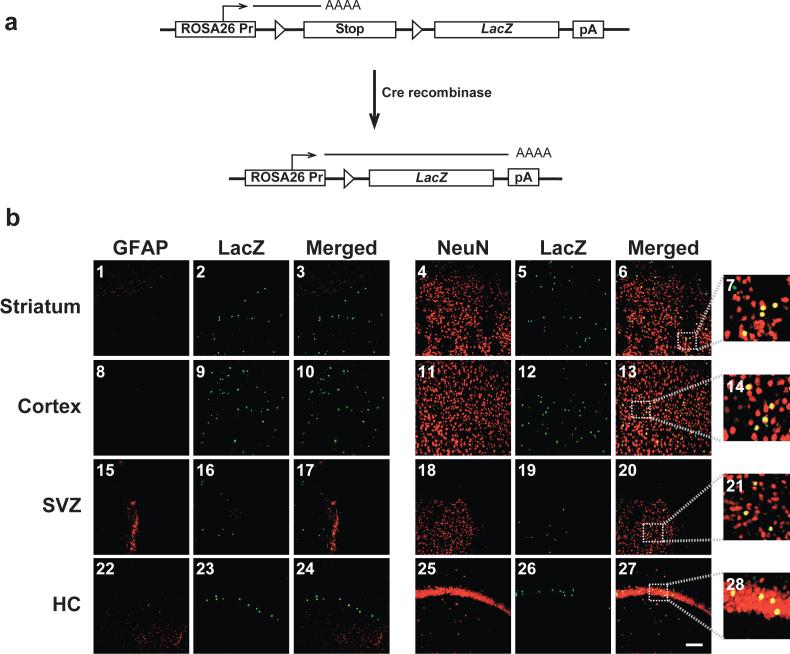
Figure 5. In utero intracerebroventricular delivery of HSVcre into E14.5 ROSA26 mouse embryos leads to Cre-mediated recombination and expression of β-galactosidase primarily in NeuN-positive neurons of the adult brain.
Staged embryonic mice were generated from mating C57BL/6 female mice with ROSA26 LacZ reporter heterozygous male mice. ROSA26 mice contain the LacZ reporter gene downstream of a transcriptional/translational stop cassette flanked by two loxP sites [12]. In a ROSA26 mouse, ectopic expression of Cre recombinase results in the juxtaposition of the ubiquitously expressed ROSA26 promoter and the LacZ gene, ultimately leading to the expression of the reporter gene product (a). ROSA26 mouse embryos intracerebroventricularly inoculated with HSVcre at E14.5 were sacrificed and perfused with 4% paraformaldehyde at 29 days of age. Coronal brain sections (30 μm) were processed for dual LacZ/NeuN or LacZ/GFAP fluorescent immunohistochemistry and imaged using confocal microscopy (b). Representative sections of the striatum (b1-b7), cortex (b8-b14), subventricular zone (SVZ; b15-b21), and hippocampus (HC; b22-b28) are shown. β-galactosidase immunopositivity, resulting from cre recombinase-mediated recombination and excision of the loxP-bound transcriptional/translational stop cassette, appears in the green channel (b2, b5, b9, b12, b16, b19, b23, and b26). Cell-type markers (GFAP and NeuN) were detected in the red channel (GFAP: b1, b8, b15, and b22; NeuN: b4, b11, b18, and b25), while co-localization is depicted in yellow (Merged: b3, b6, b10, b13, b17, b20, b24, and b27). Panels b7, b14, b21, and b28 depict 5X expanded views of the area outlined by the dotted box in Panels b6, b13, b20, and b27, respectively. Mice injected with the null vector, HSVPrPUC, did not exhibit any detectable β-galactosidase-positive cells (data not shown). Magnification bar shown in lower right panel = 50 μm (final magnification = 20X). N = 4 for each experimental group.