TABLE 1.
Formation of Allyl Enol Carbonates from Various Substituted Allyl 1H-imidazole-1-carboxylates
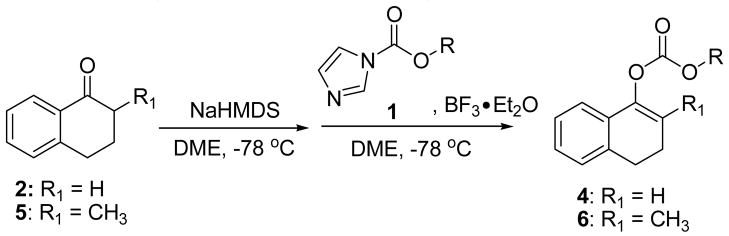 | |||||
|---|---|---|---|---|---|
| Entry | OR | Yield of 1a | Ketone | Product | Yield |
| 1 |
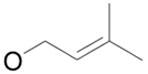
|
1b
80% |
2 | 4b | 88% |
| 2 | 5 | 6b | 99% | ||
| 3 |
|
1c
91% |
2 | 4c | 76% |
| 4 | 5 | 6c | 76% | ||
| 5 |

|
1d
97% |
2 | 4d | 80% |
| 6 | 5 | 6d | 94% | ||
| 7 |
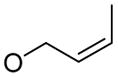
|
1e
85% |
5 | 6e | 89% |
| 8 |
|
1f
95% |
5 | 6f | 96% |
| 9 |
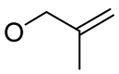
|
1g
97% |
5 | 6g | 92% |
| 10 |
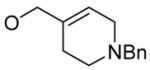
|
1h
100% |
5 | 6h | 76% |
Isolated yields of 1 from the reaction of 1,1′-carbonyldiimidazole and the corresponding allyl alcohol.
