TABLE 2.
The Reaction of Various Ketone Enolates with 1 or Its BF3 Complex
| Entry | Ketone | Procedurea | Product | Yieldb |
|---|---|---|---|---|
| 1 |
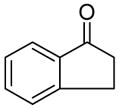
|
A |
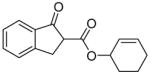
7 |
63% |
| 2 | B |
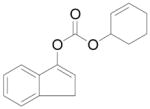
8 + 7 (8:7 = 3:1) |
95% | |
| 3 | C | 8 + 7 (17:1) | 92% | |
| 4 | D | 8 only | 92% | |
| 5 |
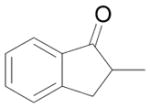
|
C |
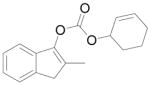
9 |
88% |
| 6 |
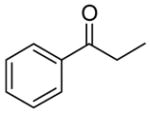
|
A |
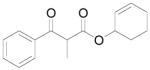
10 |
61% |
| 7 | B |
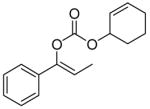
11 |
99% | |
| 8 |

|
A |
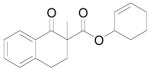
12 + 4d (12:4d = 1.3:1) |
51% |
| 9 |
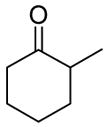
|
Bc |
|
99% |
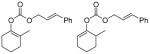
| 10 |

|
C |
|
93% |
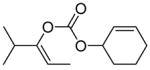
| 11 |

|
B |
|
78% |
A: 1.0 equiv. NaHMDS, DME, −78 °C, then 0.8 equiv. 1; B: 1.0 equiv. NaHMDS, DME, −78 °C, then mixture of 0.8 equiv. 1 and BF3·Et2O or, if the product can not be separated from the starting ketone by silica gel chromatography, 1.2 equiv. NaHMDS, DME, −78 °C, then mixture of 1.2 equiv. 1 and BF3·Et2O; C: 1.2 equiv. KOtBu, THF, −78 °C, then mixture of 1.2 equiv. 1 and BF3·Et2O; D: 1.2 equiv. KOtBu, 1.2 equiv. 18-crown-6, THF, −78 °C, then mixture of 1.2 equiv. 1 and BF3·Et2O.
Combined isolated yield for the reactions with more than one product.
The enolate was formed by the addition of a solution of 1 equiv. of NaHMDS into a solution of the substrate at 0 °C then cooled to −78 °C.
