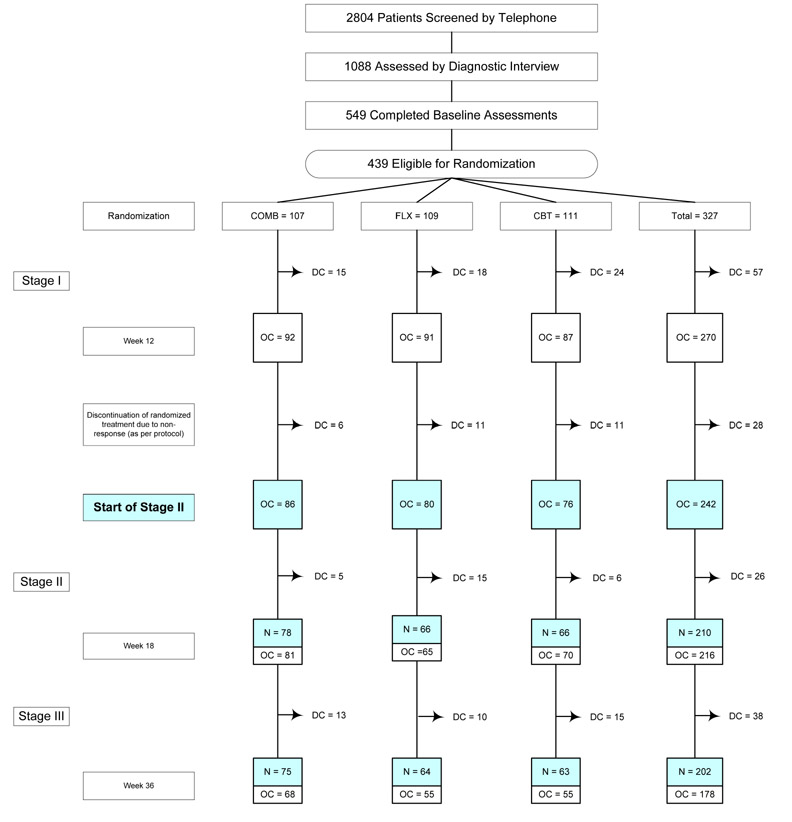
Figure 1.
Treatment for Adolescents with Depression Study (TADS): Flow diagram for the N = 242 who continued randomized treatment after 12 weeks of acute treatment.
Reasons for discontinuation prior to randomization have been previously reported (TADS Team, 2004, JAMA, p. 811). COMB = combination of fluoxetine and cognitive behavior therapy; FLX = fluoxetine; CBT = cognitive behavior therapy; PBO = pll placebo; DC = discontinuation of randomized treatment due to premature termination, non-response at the end of Stage I, or study exit; OC (observed case) = youth still in randomized treatment arm;
The 242 youths who started Stage II as an observed case were evaluated in the current analysis; N = Number of CGI-1 assessments completed for 242 cases at Week 18 and Week 36. All 112 patients assigned to the PBO condition discontinued randomized treatment at the end of Stage I as per protocol and are excluded from the current analysis.