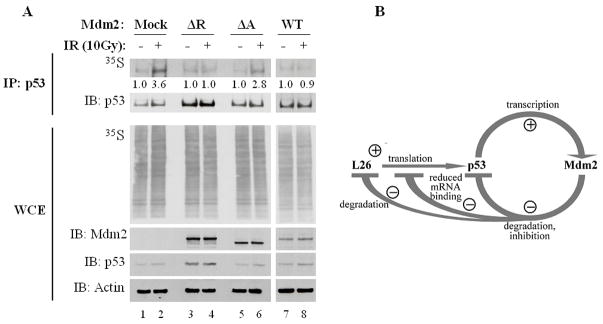
Figure 7. Excess Mdm2 inhibits DNA damage-induced enhancement of p53 translation in vivo.
A. MCF7 cells were transiently transfected with the corresponding Mdm2 expression plasmids and treated with 50μM MG132 for 30 minutes prior to exposure to 10Gy ionizing radiation (IR). Twenty five minutes after IR the cells were pulse-labeled for 5 minutes with [35S]methionine. p53 protein was immunoprecipitated (IP) and the level of immunoprecipitated radiolabeled protein was assessed by autoradiography (35S); Western blot analysis was employed to assess the total amount of immunoprecipitated p53 (IB:p53). Autoradiography of hole cell extracts (WCE, 35S, middle panel) confirm equal amounts of overall [35S]methionine incorporation into cellular proteins and Western blot analysis of WCE shows total levels of Mdm2 and p53. Numbers below upper panel indicate the relative increase in p53 synthesis upon IR treatment for each pair of samples.
B. Model depicting the involvement of L26 in the p53-Mdm2 autoregulatory loop. Transcription of the mdm2 gene is positively regulated by p53. In turn, the Mdm2 protein binds p53, inhibits its biochemical activities and targets it for ubiquitin-dependent proteasomal degradation. Translation of p53 mRNA is positively regulated by L26, but Mdm2 reduces this effect by promoting L26 degradation as well as interfering with the binding of L26 to p53 mRNA. Thus, Mdm2 downregulates p53 by both inhibiting its synthesis and augmenting its demise.