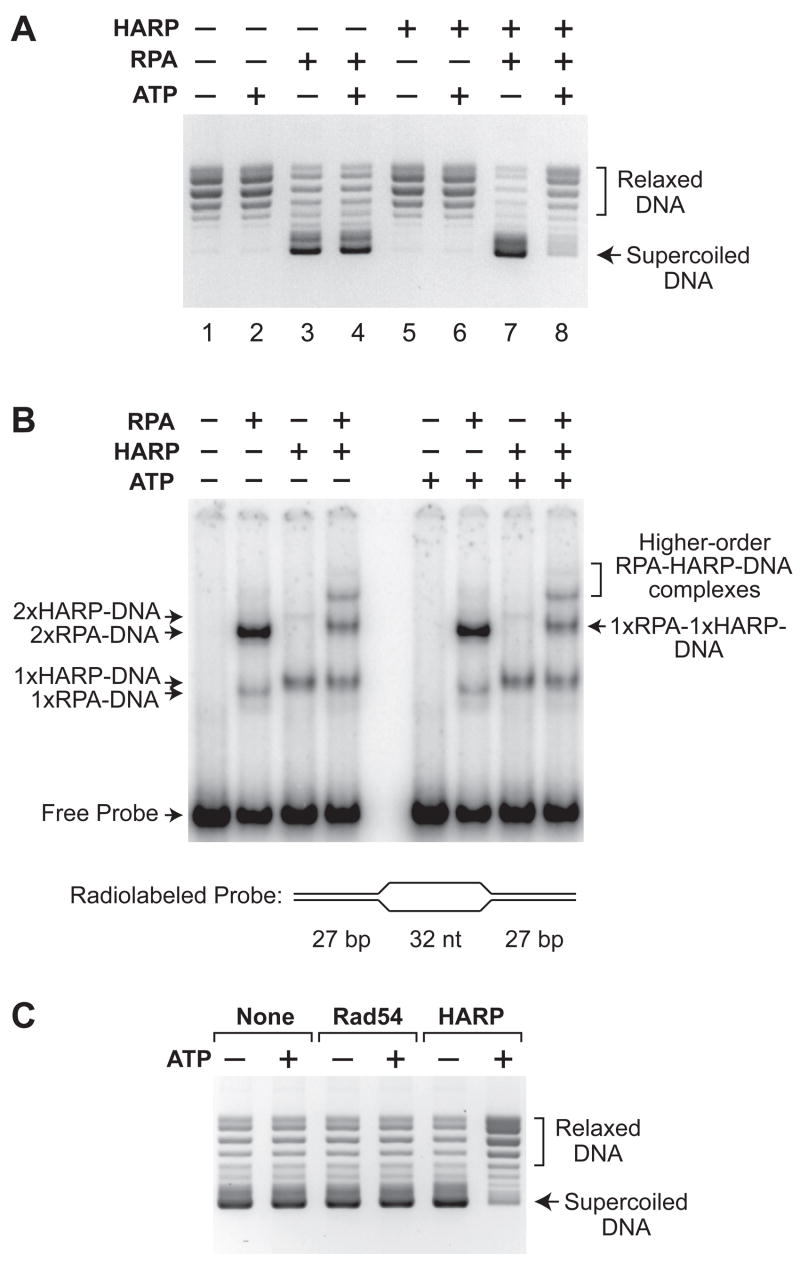
Fig. 2.
HARP is an ATP-dependent annealing helicase. (A) HARP catalyzes the rewinding of DNA in an ATP-dependent manner. Annealing helicase assays, as depicted in fig. S3, were carried out in the presence or absence of the indicated factors, and the resulting DNA species were resolved by agarose gel electrophoresis. An equimolar concentration of UTP was used as a control for the absence of ATP. (B) HARP does not catalyze the ATP-dependent displacement of RPA from DNA. Gel mobility shift experiments were performed with radiolabeled bubble DNA that contains two high affinity sites for RPA (the two 32 nt single-stranded DNA segments) and for HARP (the two DNA forks). HARP (2 nM), RPA (3 nM), and ATP (1.5 mM) were included, as indicated. The apparent compositions of the shifted complexes are specified. Quantitation of the bands is shown in fig. S5. (C) Rad54, a member of the SNF2 family that translocates along DNA, does not exhibit annealing helicase activity. Annealing helicase assays were performed as in A with RPA and an equimolar concentration of Rad54 or HARP, where indicated.