Fig. 1.
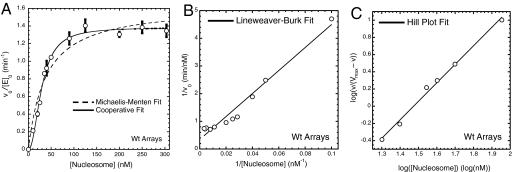
Acetylation of nucleosomal arrays by the SAGA complex is cooperative with respect to array concentration. (A) Initial velocity of nucleosomal array acetylation per enzyme as a function of nucleosome concentration, where [SAGA]0 = 1.0 nM and [acetyl-CoA]0 = 4.0 μM. For each nucleosomal array concentration the initial velocity is the average of 3 trials, with the associated SD indicated by the error bars. These data points were fit to both a Michaelis–Menten model (dashed line) and a cooperative saturation kinetics model (solid line). The cooperative saturation fit gives a cooperativity constant of 1.97 ± 0.15. (B) Linear fit of the data from A by using the Lineweaver–Burk formulation of the Michaelis–Menten model. R = 0.983. (C) Linear fit of the data from A by using the Hill plot formulation of the cooperative saturation kinetics model. Data were fit where the Hill plot demonstrates linearity, from 10 to 90% saturation (34). Cooperativity constant is 1.91 ± 0.08. R = 0.997.