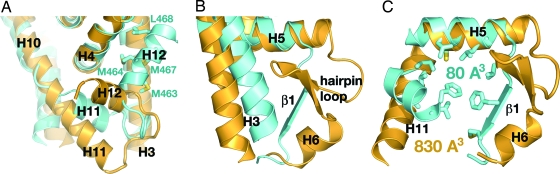
Fig. 3.
Structural comparison of LRH-1 (yellow) and Dax-1 (blue) LBDs. (A) The AF-2 sites of LRH-1 and Dax-1. Helix H12 of LRH-1 is in an “active” conformation, whereas helix H12 of Dax-1 is in an “inactive” conformation and is docked in the coactivator groove; the residues participating in the docking interactions are indicated. Helix H11 of Dax-1 is rotated by ≈45° compared with its counterpart in LRH-1. (B) Helices H3 and H5-H6 of LRH-1 and Dax-1. Helix H3 of Dax-1 is shifted toward the core of the LBD. Helix H5 of Dax-1 is shorter and is followed by a short β-strand (indicated). (C) The ligand-binding pockets of LRH-1 and Dax-1. Residues filling the putative ligand-binding cavity of Dax-1 are shown as stick models.