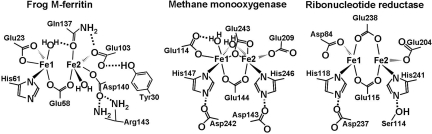
Fig. 1.
Comparisons of diiron sites for Fe substrate (ferritin) and cofactors reveal significant differences in iron ligands and second-shell residues. The ferritin Fe2 site ligands are Glu103, Gln137, and Asp140, with a bridging carboxylate, Glu58. The distinctive ferritin Gln137 is compared with Glu243 in methane monooxygenase (MMO) and Glu238 in ribonucleotide reductase (RNR). Multiple histidine bonding interactions in diiron cofactor centers are absent from ferritin diiron substrate centers. The figure uses the frog M ferritin model [Protein Data Bank (PDB) file 1MFR, a crystal structure with Mg(II) as an Fe(II) homologue (18)] and CD/MCD analysis of diferrous binding (17). Diiron cofactor site structures include diferrous, as in MMO (1FYZ) (21) and RNR (1PFR) (22).