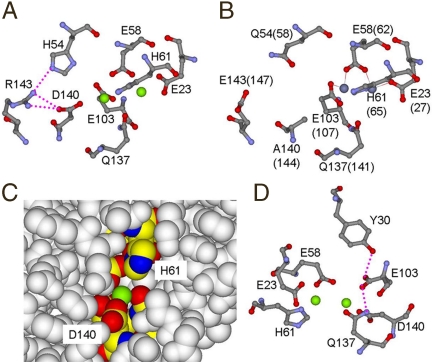
Fig. 3.
Models of the environments around ferritin Fe2 site related to Fe(II)/O2 oxidoreductase activity. (A) The Fe2 environment with Asp at variable position 140. Arg143 hydrogen-bonded to Asp140 and His54, near Asp140, covary among ferritins. (B) The environment of variable residue with Ala140(144); same view as C. The hydrogen-bond network Arg143···His54 would be absent with Glu143 and Gln54. [The figure was created from PDB 1MFR, frog M-ferritin, for Asp140, His54, Arg 143 Mg(II) cocrystals (18) and PDB 2CEI, Ala140(144), Glu143(147), and Gln54(58), human H-ferritin Zn(II) cocrystals (35).] Dotted lines indicate presumed hydrogen bonds. (C) The inner cavity surface around the catalytic center. Fe2 site Asp140 is located at the inner cavity surface; Mg(II) (green) is exposed to cavity solvent. (D) A plausible hydrogen bonding network: the invariant ferritin Fe2 amide side chain of Gln137 hydrogen-bonds to the carboxylate group of conserved Glu103, which is hydrogen-bonded with the conserved Tyr30 phenoxyl.