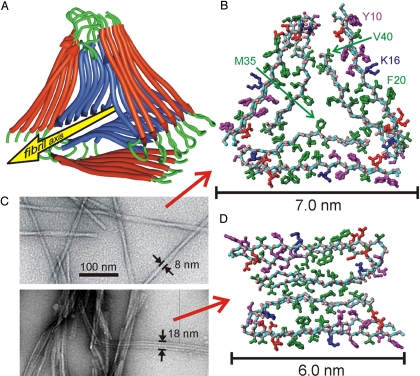
Fig. 4.
Experimentally based structural models. (A) Ribbon representation of the lowest-energy model for fibrils with the twisted morphology in Fig. 1. Modeling calculations assume threefold symmetry, consistent with STEM data and solid state NMR spectra, and are constrained by specific secondary, tertiary, and quaternary structural data from solid state NMR. (B) Atomic representation, viewed down the fibril axis. Hydrophobic, polar, negatively-charged, and positively charged amino acid sidechains are green, magenta, red, and blue, respectively. Backbone nitrogen and carbonyl oxygen atoms are cyan and pink. Unstructured N-terminal residues 1–8 are omitted. (C) Comparison of twisted (Upper) and striated ribbon (Lower) fibril morphologies in negatively stained TEM images. (D) Atomic representation of a model for striated ribbon fibrils developed previously by Petkova et al. (4, 15).