Abstract
Scaffolds represent important components for tissue engineering. However, researchers often encounter an enormous variety of choices when selecting scaffolds for tissue engineering. This paper aims to review the functions of scaffolds and the major scaffolding approaches as important guidelines for selecting scaffolds and discuss the tissue-specific considerations for scaffolding, using intervertebral disc as an example.
Keywords: Tissue engineering, Scaffolding, Scaffolds, Biomaterials, Intervertebral disc
Introduction
Since its emergence in the mid-1980s, tissue engineering has continued to evolve as an exciting and multidisciplinary field aiming to develop biological substitutes to restore, replace or regenerate defective tissues [59, 64]. Cells, scaffolds and growth-stimulating signals are generally referred to as the tissue engineering triad, the key components of engineered tissues. Scaffolds, typically made of polymeric biomaterials, provide the structural support for cell attachment and subsequent tissue development. However, researchers often encounter an enormous variety of choices when selecting scaffolds for tissue engineering. Hence, this paper reviews the functions of scaffolds, the scaffolding approaches and the tissue-specific considerations for scaffolding, using intervertebral disc as an example.
Analogous functions of scaffolds and extracellular matrix
Apart from blood cells, most, if not all other, normal cells in human tissues are anchorage-dependent residing in a solid matrix called extracellular matrix (ECM). There are numerous types of ECM in human tissues, which usually have multiple components and tissue-specific composition. Readers are directed to detailed reviews for types of ECM [9, 90, 95] and their tissue-specific composition [13, 91, 112]. As for the functions of ECM in tissues, they can be generally classified into five categories (Table 1). Firstly, ECM provides structural support and physical environment for cells residing in that tissue to attach, grow, migrate and respond to signals. Secondly, ECM gives the tissue its structural and therefore mechanical properties, such as rigidity and elasticity that is associated with the tissue functions. For example, well-organized thick bundles of collagen type I in tendon are highly resistant to stretching and are responsible for the high tensile strength of tendons. On the other hand, randomly distributed collagen fibrils and elastin fibers of skin are responsible for its toughness and elasticity. Thirdly, ECM may actively provide bioactive cues to the residing cells for regulation of their activities. For examples, the RGD sequence on fibronectin triggers binding events [47] while the regular topological pattern stimulates preferred alignment of cells [26, 127]. Fourthly, ECM may act as reservoir of growth factors and potentiate their bioactivities. For example, heparin sulfate proteoglycans facilitate bFGF dimerization and thus activities [102]. Fifthly, ECM provides a degradable physical environment so as to allow neovascularization and remodeling in response to developmental, physiological and pathological challenges during tissue dynamic processes namely morphogenesis, homeostasis and wound healing, respectively.
Table 1.
Functions of extracellular matrix (ECM) in native tissues and of scaffolds in engineered tissues
| Functions of ECM in native tissues | Analogous functions of scaffolds in engineered tissues | Architectural, biological, and mechanical features of scaffolds | |
|---|---|---|---|
| 1. | Provides structural support for cells to reside | Provides structural support for exogenously applied cells to attach, grow, migrate and differentiate in vitro and in vivo | Biomaterials with binding sites for cells; porous structure with interconnectivity for cell migration and for nutrients diffusion; temporary resistance to biodegradation upon implantation |
| 2. | Contributes to the mechanical properties of tissues | Provides the shape and mechanical stability to the tissue defect and gives the rigidity and stiffness to the engineered tissues | Biomaterials with sufficient mechanical properties filling up the void space of the defect and simulating that of the native tissue |
| 3. | Provides bioactive cues for cells to respond to their microenvironment | Interacts with cells actively to facilitate activities such as proliferation and differentiation | Biological cues such as cell-adhesive binding sites; physical cues such as surface topography |
| 4. | Acts as the reservoirs of growth factors and potentiates their actions | Serves as delivery vehicle and reservoir for exogenously applied growth-stimulating factors | Microstructures and other matrix factors retaining bioactive agents in scaffold |
| 5. | Provides a flexible physical environment to allow remodeling in response to tissue dynamic processes such as wound healing | Provides a void volume for vascularization and new tissue formation during remodeling | Porous microstructures for nutrients and metabolites diffusion; matrix design with controllable degradation mechanisms and rates; biomaterials and their degraded products with acceptable tissue compatibility |
Intuitively, the best scaffold for an engineered tissue should be the ECM of the target tissue in its native state. Nevertheless, the multiple functions, the complex composition and the dynamic nature of ECM in native tissues make it difficult to mimic exactly. Therefore, contemporary concept of scaffolding in tissue engineering is to mimic the functions of native ECM, at least partially. As a result, the important roles played by scaffolds in engineered tissues, as reviewed elsewhere [79, 109], are analogous to the functions of ECM in native tissues and are associated with their architectural, biological, and mechanical features (Table 1). Let us consider these functions and features as follows:
Architecture: Scaffolds should provide void volume for vascularization, new tissue formation and remodeling so as to facilitate host tissue integration upon implantation. The biomaterials should be processed to give a porous enough structure for efficient nutrient and metabolite transport without significantly compromising the mechanical stability of the scaffold. Moreover, the biomaterials should also be degradable upon implantation at a rate matching that of the new matrix production by the developing tissue.
Cyto- and tissue compatibility: Scaffolds should provide support for either extraneously applied or endogenous cells to attach, grow and differentiate during both in vitro culture and in vivo implantation. The biomaterials used to fabricate the scaffolds need to be compatible with the cellular components of the engineered tissues and endogenous cells in host tissue.
Bioactivity: Scaffolds may interact with the cellular components of the engineered tissues actively to facilitate and regulate their activities. The biomaterials may include biological cues such as cell-adhesive ligands to enhance attachment or physical cues such as topography to influence cell morphology and alignment. The scaffold may also serve as a delivery vehicle or reservoir for exogenous growth-stimulating signals such as growth factors to speed up regeneration. In this regard, the biomaterials need to be compatible with the biomolecules and amenable to an encapsulation technique for controlled release of the biomolecules with retained bioactivity. For example, hydrogels synthesized by covalent or ionic crosslinking can entrap proteins and release them by a mechanism controlled by swelling of the hydrogels [11].
Mechanical property: Scaffolds provide mechanical and shape stability to the tissue defect. The intrinsic mechanical properties of the biomaterials used for scaffolding or their post-processing properties should match that of the host tissue. Recent studies on mechanobiology have highlighted the importance of mechanical properties of a scaffold on the seeded cells. Exerting traction forces on a substrate, many mature cell types, such as epithelial cells, fibroblasts, muscle cells, and neurons, sense the stiffness of the substrate and show dissimilar morphology and adhesive characteristics [35]. This mechanosensitivity has also been demonstrated in the differentiation of MSC [36], when stiffness of the agarose gel would determine the differentiation tendency. The hMSC would differentiate along the neuronal, muscle, or bone lineages according to stiffness that approximate those of the brain, muscle, and bone tissues, respectively.
Scaffolding approaches in tissue engineering
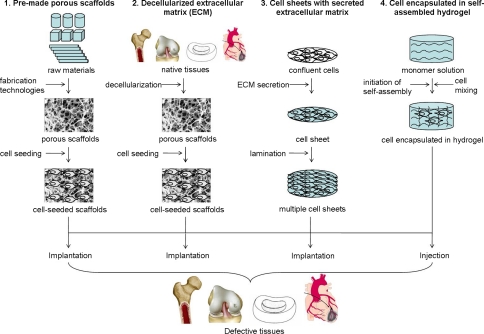
Over the last two decades, four major scaffolding approaches for tissue engineering have evolved (Fig. 1). It would be informative to highlight the working principles and the characteristics of these approaches (Table 2) before discussing the tissue-specific considerations.
Fig. 1.
Schematic diagram showing different scaffolding approaches in tissue engineering
Table 2.
Characteristics of different scaffolding approaches in tissue engineering
| Scaffolding approach | (1) Pre-made porous scaffolds for cell seeding | (2) Decellularized extracellular matrix for cell seeding | (3) Confluent cells with secreted extracellular matrix | (4) Cell encapsulated in self-assembled hydrogel |
|---|---|---|---|---|
| Raw materials | Synthetic or natural biomaterials | Allogenic or xenogenic tissues | Cells | Synthetic or natural biomaterials able to self-assemble into hydrogels |
| Processing or fabricating technology | Incorporation of porogens in solid materials; solid free-form fabrication technologies; techniques using woven or non-woven fibers | Decellularization technologies | Secretion of extracellular matrix by confluent cells | Initiation of self-assembly process by parameters such as pH and temperature |
| Strategy to combine with cells | Seeding | Seeding | Cells present before extracellular matrix secretion | Cells present before self-assembly |
| Strategy to transfer to host tissues | Implantation | Implantation | Implantation | Injection |
| Advantages | Most diversified choices for materials; precise design for microstructure and architecture | Most nature-simulating scaffolds in terms of composition and mechanical properties | Cell-secreted extracellular matrix is biocompatible | Injectable, fast and simple one-step procedure; intimate cell and material interactions |
| Disadvantages | Time consuming cell seeding procedure; inhomogeneous distribution of cells | Inhomogeneous distribution of cells, difficulty in retaining all extracellular matrix, immunogenicity upon incomplete decellularization | Need multiple laminations | Soft structures |
| Preferred applications | Both soft and hard tissues; load-bearing tissues | Tissues with high ECM content; load-bearing tissues | Tissues with high cellularity, epithelial tissues, endothelial tissues, thin layer tissues | Soft tissues |
Pre-made porous scaffolds for cell seeding
Since the birth of “tissue engineering” [65, 114], seeding therapeutic cells in pre-made porous scaffolds made of degradable biomaterials has become the most commonly used and well-established scaffolding approach. This approach represents the bulk of biomaterial research in tissue engineering, leading to enormous efforts in development of different types of biomaterials and fabrication technologies.
Many types of biomaterials can be used to make porous scaffolds for tissue engineering provided that a fabrication technology compatible with the biomaterial properties is available. Reviewing these biomaterials in detail is out of the scope of this paper and readers are directed to other reviews [51, 61, 92]. In general, biomaterials used for making porous scaffolds for tissue engineering can be classified into two categories according to their sources, namely natural and synthetic biomaterials. Naturally occurring biomaterials can be obtained from their natural sources and processed to make porous scaffolds. These materials can be in their native form, such as ECM from allografts and xenografts, or can be in the form of smaller building blocks, which include but not limited to inorganic ceramics such as calcium phosphates and organic polymers such as proteins, polysaccharides, lipids and polynucleotides. Natural biomaterials usually have superb biocompatibility so that cells can attach and grow with excellent viability. However, one issue with natural materials is their limited physical and mechanical stability and therefore they may not be suitable for some load-bearing applications. This is the reason why researchers using natural biomaterials are prompted to develop technologies improving and reinforcing the mechanical and shape stability of natural biomaterials. Examples include developing composites with synthetic material [14] and crosslinking [20, 22, 55]. Another issue is the potential immunogenicity, because natural biomaterials from allogenic or xenogenic sources may be antigenic to the hosts. As a result, researchers are attracted to technologies such as removal of telopeptides in procollagen [100] for reduction of immunogenicity. Synthetic biomaterials can be generally categorized into inorganic such as bioglasses, and organic such as synthetic polymers. It is generally believed that synthetic biomaterials have better controlled physical and mechanical properties and can be used to tailor for both soft and hard tissues. Nevertheless, for synthetic biomaterials, biocompatibility becomes the major issue because cells may have difficulties in attachment and growth on these materials. Therefore, many processes modifying the surface and bulk properties have been developed to improve their biocompatibility [78]. Examples include surface laser engineering [63] and coating with natural biomaterials such as collagen [16]. With the development of composites materials, the combinations of biomaterials for making porous scaffolds have become enormous. Multiple types of biomaterials with distinct properties can be blended and fabricated to be tailored for a particular application.
Over the last decade, development of fabrication technologies for porous scaffolds has been an intensive area of research. Readers are also directed to several excellent review articles for various fabrication technologies [25, 34, 50, 54, 107, 109, 124, 125]. In general, these technologies can be classified into (1) processes using porogens in biomaterials, (2) solid free-form or rapid prototyping technologies and (3) techniques using woven or non-woven fibers. In the first category [25], solid materials either in solids or dissolved in solvents, are incorporated with porogens, which could be gases such as carbon dioxide, liquids such as water or solids such as paraffin. The mixtures are processed and then cast or extruded. Porogens are removed after fabrication using methods such as sublimation, evaporation and melting to leave behind a porous structure in the scaffold. Example techniques include solvent casting and particulate leaching, gas foaming, freeze-drying and phase separation [124]. In the second category, hierarchical porous structures are manufactured by sequential delivery of material and/or energy needed to bond the materials to preset points in space [50]. Some solid free-form fabrication technologies [34, 50, 54] such as selective laser sintering, stereolithography and 3D printing depend on precise delivery of light or heat energy in a scanner system to points of space in the material bed so as to bond or crosslink the materials to give solid structures in an otherwise soluble bed of materials. Some solid free-form fabrication technologies [50] such as wax printing rely on simultaneous delivery of solid materials and removable supporting materials in a layer-by-layer printing manner. In the third category, woven and non-woven fiber structures can be piled together and bonded using thermal energy or adhesives to give a porous meshwork using techniques such as fiber bonding [124], or fibers can be generated by the electrospinning technique, in which a high voltage is injected to a polymer solution where the electrostatic forces are built up to overcome the surface tension of the polymer solution and therefore form a spinning fiber jet [18, 26, 27, 32, 89, 107].
This scaffolding approach using pre-made porous scaffolds has a number of advantages. Firstly, this approach has the most diversified choice for biomaterials. Almost all biomaterials ranged from ceramics to hydrogels have suitable fabrication technologies. Secondly, relatively precise designs on architecture and microstructure of the scaffolds can be incorporated by this approach. Therefore, the physicochemical properties of the porous scaffolds are easily engineered in order to mimic the physical properties of native ECM in the target tissues. This is especially advantageous for making load-bearing tissues where the mechanical properties are important. Nevertheless, this approach also has certain disadvantages. In particular, post-fabrication cell-seeding to porous scaffolds is time-consuming and inefficient because of the limited penetration ability of cells into the scaffolds. This usually leads to inhomogeneous distribution of cells in the scaffolds and therefore heterogeneous properties in the engineered tissues. As a result, various efforts such as agitation, perfusion and enlarged pore size are needed to enhance cell seeding efficiency and these methods are not free of problems, to list a few, low cell viability and high cost.
Decellularized ECM from allogenic or xenogenic tissues for cell seeding
Acellular ECM processed from allogenic or xenogenic tissues are the most nature-simulating scaffolds, which have been used in tissue engineering of many tissues including heart valves [62], vessels [15], nerves [44], tendon and ligament [56]. This scaffolding approach removes the allogenic or xenogenic cellular antigens from the tissues as they are the sources for immunogenicity upon implantation but preserves the ECM components, which are conserved among species and therefore well tolerated immunologically. Specialized decellularization techniques are developed to remove cellular components and this is usually achieved by a combination of physical, chemical and enzymatic methods [9, 40]. In brief, cell membranes are lysed by physical treatments such as freeze-thaw cycles or ionic solutions such as hypo or hypertonic solutions before separating the cellular components from the ECM by enzymatic methods such as trypsin/EDTA treatment. The cytoplasmic and nuclear cellular components will then be solubilized and removed by a handful choice of detergents. The process parameters need to be optimized to aid complete decellularization with minimal disturbance on biochemical composition and mechanical properties of the ECM. Decellularized ECM can be used for homologous functions when the decellularized ECM is used to replace an analogous structural tissue that has been damaged. An example is to decellularize vessels as allogenic vascular grafts [15, 101]. Decellularized ECM can also be used for non-homologous functions when it is used for a purpose different from which it fulfills in its native state, or in a location of the body where such structural function does not normally occur. Examples are to use small intestinal submucosa (SIS) for vascular graft, tendon, dura mater, skin and other tissues [7, 8, 38, 49, 101] and to use amnion membrane for peripheral nerve regeneration [77]. The major advantage of this scaffolding approach is the most close-to-nature mechanical and biological properties of the decellularized ECM. Moreover, apart from the excellent biocompatibility of the natural ECM, growth factors preserved in the decellularized matrix may further facilitate cell growth and remodeling [9]. Nevertheless, cell seeding in decellularized ECM may also lead to inhomogeneous distribution while incomplete removal of cellular components may elicit immune reactions upon implantation [129].
Cell sheets with self-secreted ECM
Cell sheet engineering represents an approach where cells secrete their own ECM upon confluence and are harvested without the use of enzymatic methods. This is achieved by culturing cells on thermo-responsive polymer, such as poly(N-isopropylacrylamide) coated culture dish until confluence. The confluent cell sheet is then detached by thermally regulating the hydrophobicity of the polymer coatings without enzymatic treatment. Such approach can be repeated to laminate multiple single cell layers to form thicker matrix. This technology is pioneered by Japanese group [84, 85, 110] and has been systemically applied to a number of applications such as cornea [82] in clinical trials and myocardium [106] in preclinical trials. The same approach has been modified and improved to produce patterned substrates for thicker tissue fabrication and for injectable applications [57]. Thermosensitive chitosan has also been investigated to create an aligned cell sheet [32]. Cell sheet engineering approach is excellent for epithelium, endothelium and cell-dense tissues [111, 123] as the formation of cell sheets require cells to grow into confluence at high density such that cells can form tight junctions with each other and secrete ECM of their own. High cell density and close association is a characteristic of epithelium and endothelium. Therefore, corneal epithelium and endothelium, vessel endothelium and tracheal epithelium are good candidates of this approach. Another advantage of the cell sheet engineering approach is that the laminated layers resulted in rapid neovascularization unlike transplantation of thick constructs with cell-seeded scaffolds. A recent report reviewed other advantages of the cell sheet engineering approach such as easy harvesting procedure, possibility of sutureless transplantation, and even distribution of cells in the sheet without mass transfer problem [31]. Nevertheless, one disadvantage of this approach is that it is difficult to construct thick tissues as each layer is around 30 μm thick. Multisurgeries such as 10 layers to form a 300 μm thick myocardial patch are required [104, 105]. This is less clinically feasible as multiple surgeries in patients are unlikely and therefore preformed patches are needed. The other disadvantage or perhaps limitation of cell sheet engineering approach is that constructing ECM rich tissues and hypocellular tissue such as bones, cartilage and intervertebral disc are unlikely as the amount of ECM secreted upon cell confluence is severely limited. In another word, tissues with rich ECM for load bearing purposes are unlikely to be fabricated by a cell sheet engineering approach [123].
Cell encapsulation in self-assembled hydrogel matrix
Encapsulation is a process entrapping living cells within the confines of a semi-permeable membrane or within a homogenous solid mass [66, 86, 87, 113]. The biomaterials used for encapsulation are usually hydrogels, which are formed by covalent or ionic crosslinking of water-soluble polymers. Many types of biomaterials including natural and synthetic hydrogels can be used for encapsulation provided that the conditions inducing the hydrogel formation or the polymerization are compatible with living cells. Encapsulation has been developed over several decades and the predominating use is for immunoisolation during allogenic or xenogenic cell transplantation [87, 113]. Naturally occurring polysaccharides derived from algae, sodium alginate is the most commonly used material while other natural materials such as agarose [10] and chitosan [130] and synthetic materials such as poly (ethylene glycol) (PEG) [83] and polyvinylalcohol (PVA) [58] are also used. The most well-known application is xenogenic pancreatic cell transplantation for diabetes [41, 66] while applications for other disorders such as CNS insufficiency [86] and liver failure [24] are also reported. For immunoisolation to work, biomaterials encapsulating the cells need to be crosslinked or processed to become impenetrable to cells, impermeable to large molecules such as antibodies and cellular antigens but permeable to nutrients such as oxygen and glucose, metabolites such as carbon dioxide and lactic acid, and secreted therapeutic biomolecules from the encapsulated cells such as insulin from pancreatic beta cells. In case of a semi-permeable membrane, the encapsulated cells should have the ability to maintain viability and functionality in aggregates even though there is only solid anchorage support limited to the luminal surface. Pancreatic cells, hepatocytes and haematopoietic cells are of this type. In case of a homogenous solid mass, where the entrapped cells are closely interacting with the biomaterials, the biomaterials should have good biocompatibility enabling cellular attachment and growth. Nevertheless, the commonly used encapsulating materials such as alginate and agarose have limited ability to support cell attachment growth and differentiation, resulting in low cell viability and growth [42, 83, 131]. In many cases, a biomaterial with better biocompatibility such as collagen must be supplemented for improvement in cell viability [10, 42]. Collagen is a natural biocompatible and biodegradable material [126] and can be reconstituted into fibrous structures simulating the native ECM in tissues. Recently, a microencapsulation system immobilizing living cells within reconstituted collagen fiber meshwork has been established [19] and the collagen meshwork is able to provide a bio-mimetic scaffold supporting cell growth, migration [21], therapeutic protein secretion [122] and stem cell differentiation [52]. Moreover, chemical approaches have been used to design self-assembled peptides [39, 128] and these biomimetic peptides can also be used to entrap cells [117]. One important feature of encapsulation is that the biomaterials used are able to self-assemble from liquid monomers to solid polymer meshwork upon initiation, which is usually pH, temperature, ionic strength and light controlled. To list a few examples, alginate solidifies when its monomer solution is exposed to divalent ion solutions such as calcium chloride, where the calcium ion crosslinks the alginate; collagen monomers polymerize when they are switched from an acidic pH and a low temperature to a neutral pH and a body temperature; ethylene glycol modified with acrylic moiety starts to polymerize when it is exposed to UV light in the presence of a photo-initiator [81]. This unique feature combines the scaffold fabrication and the cell seeding into one-step procedure as cells can be mixed with the liquid biomaterials before initiation of polymerization. The advantages of this approach include simple one-step procedure, homogenous cell distribution in the hydrogel and excellent cell viability. Moreover, this self-assembled approach enables injectable application where the polymerization can be initiated after injection, leading to “setting” of the hydrogel after they are injected into the defective tissues. This represents a minimally invasive approach of tissue engineering and is advantageous when the defect is irregularly shaped. Because of the injectable feature of the scaffold, this approach is sometimes referred as injectable scaffolding or in situ tissue engineering. Nevertheless, hydrogel materials in this approach used usually have poor mechanical properties. As a result, this scaffolding approach is seldom used for tissues with load bearing functions.
Scaffolding in intervertebral disc tissue engineering
Selecting the scaffolding approach for tissue engineering is tissue- and application-specific. Intervertebral disc is chosen as an illustrating example in this review. Extensive efforts have been made to search for biological therapeutics for disc degeneration of different severity [48, 103]. Readers are directed to excellent reviews on structural functional relationship and pathophysiological aspects of IVD [1, 12, 116] and excellent reviews on potential biological therapies including growth factor, cell and tissue engineering approaches [3, 6, 37, 43, 88]. In this review, existing scaffolding approaches for disc regeneration and their insufficiencies, the unique considerations of intervertebral disc and the future directions of scaffolding in IVD tissue engineering will be reviewed (Table 3).
Table 3.
Existing scaffolding approaches, insufficiencies and future directions for IVD tissue engineering
| Stage of disc degeneration | Existing scaffolding approaches | Insufficiencies | Proposed future directions |
|---|---|---|---|
| Early degeneration | Injection of cells with and without hydrogel carriers (Approach 4) | Poor engraftment and viability | Improved viscosity and stiffness of carriers to reduce leakage and extrusion; welding or bioglue techniques to prevent leakage and extrusion |
| Mid-stage degeneration | Implantation of cell-seeded pre-made porous scaffolds (Approach 1) Implantation of cell-seeded decellularized ECM (Approach 2) Injection of cells with hydrogel carriers to replace loss in matrix (Approach 4) | Lack of in vivo model; extrusion of implants under loading | Welding or bioglue techniques to prevent leakage and extrusion; technologies to enhance the mechanical properties of scaffolds without compromising the cell viability |
| Late degeneration | Allogenic whole disc for total disc replacement; (Approach 2) Building interface between multiple tissue components | Short-term maintenance of disc height and hydration; degeneration of allograft in long term; lack of remodeling cells | Better graft preservation technologies to maintain matrix composition; scaffolds providing appropriate microenvironment for cells to remodel upon mechanical stimulationImprovement of proteoglycan retention in scaffolds for nucleus replacements; improvement of scaffold mechanical properties for stable annulus replacements; combinatory approach for IVD scaffolding; engineering of stable tissue interfaces |
In early disc degeneration, disc cells in particular the nucleus pulposus (NP) cells become less capable to synthesize the proper ECM. The gelatinous NP becomes more fibrous with reduced water content. The primary to be repaired should be the capability of the NP cells to secrete the right matrix components in particular proteoglycans, which are responsible for the water absorbing function of the nucleus matrix. At this early stage, matrix loss is still limited or partial and there is no need to replace the bulk matrix. As a result, minimally invasive treatments such as injecting growth factors to stimulate the NP cells to synthesize proteoglycans [74] or injecting cells able to synthesize appropriate ECM such as bone marrow mesenchymal stem cells [48, 67, 108] are the most commonly proposed. Nevertheless, one important inadequacy of this approach is that the high disc pressure and the aqueous nature of the cell suspension usually result in depletion of the local availability of cells [5, 53]. As a result, use of injectable carriers is necessary to effectively deliver cells into the degenerative NP space. Therefore, self-assembled hydrogels able to suspend and deliver cells in solution via injection but which can solidify after injection presents an appropriate scaffolding approach. An example is to deliver mesenchymal stem cells in hyaluronan gel [30]. Nevertheless, these hydrogel carriers still have insufficient viscosity and stiffness resulting in immediate loss of the majority of injected cells (>96%) due to back-flow via the injection path [30]. This has been observed in our own group using other hydrogels (unpublished work). Therefore, better injectable approaches such as carriers with higher viscosity and stiffness, and in situ welding techniques such as laser welding, photochemical welding and use of biological glues at the injection site should be explored in order to prevent leakage and extrusion and thus improve the effectiveness of the injectable therapy.
As the disc degeneration progresses, more ECM and structural changes such as proteoglycan and collagen degradation involving NP and sometimes annulus fibrosus (AF) ensue, leading to disc height reduction. At this stage, replacement of cells with capacity to synthesize ECM and compensation of the substantial loss of matrix components are appropriate therapeutic approaches. This can be achieved by implanting cell-seeded pre-made scaffolds (approach 1) or decellularized ECM (approach 2) or injecting cells encapsulated in self-assembled hydrogels (approach 4) into the intradiscal space. In reviewing the existing scaffolding approaches for IVD tissue engineering, approach 1 using pre-made scaffold is most common. A wide range of biomaterials dominated by natural biomaterials has been used for nucleus replacement. These materials include collagen [121], atellocollagen [98, 99], alginate [70, 76], gelatin [118], chitosan [33], collagen/glycosaminoglycan [97], collagen/hyaluronan [2] and poly-l-lactic acid [94]. Reviews for nucleus replacements have been reported elsewhere [29, 103]. On the other hand, biomaterials used for annulus replacement are dominated by synthetic biomaterials including silk [23], polycaprolactone [80] and its derivatives [118], polyglycolic acid/polylactic acid [76] and bioglass [120] with a few natural biomaterials such as collagen/hyaluronan [2]. In most if not all cases, freeze-drying has been employed as the fabrication method to create porous structures. In most nucleus and annulus replacement strategies, survival and growth of seeded cells, and enhanced synthesis of collagen II and proteoglycans by these cells are reported [2, 23, 70, 76, 94, 97, 118, 120]. Nevertheless, almost all studies using the pre-made scaffold approach are in vitro while only a few are in vivo [76]. Subcutaneous implantation was used and the implant was never exposed to physiological loading. Recently, there is one ex vivo study reporting collagen nucleus replacement in bovine discs under mechanical loading [121]. Although restoration of disc height is possible, extrusion of the whole implant after a few loading cycles have been reported. As a result, maintaining annulus integrity by sealing or welding methods after nucleotomy or injection or insertion of nucleus replacements is crucial. As for annulus replacement, the intrinsic mechanical properties of the annulus replacements have to be comparable to that of the native disc in order to be able to resist physiological loading. This is extremely challenging because the native annulus fibrosis serves a highly demanding and complex mechanical function. In existing literature, only a few annulus replacement strategies mentioned enhanced mechanical properties using composites materials [118] and electrospun fibers with alignment [80]. As a result, further efforts in enhancing the mechanical integrity of scaffolds for annulus replacement should be encouraged.
The efforts using the second scaffolding approach, decellularized ECM for nucleus replacement is minimal. Only one report uses porcine SIS as the scaffold for human disc cells [68]. Cell survival and enhanced matrix deposition have been reported in vitro [68]. In a pilot animal study using SIS in nucleotomized baboons [69], MRI suggested a higher water content in the treatment groups compared to the nucleotomy group and some tissue remodeling in the disc space in the group with bone marrow-soaked SIS. Moreover, SIS has good tissue biocompatibility in the disc space and seemed to have biodegraded over the six-month period. Nevertheless, a sizable study with more animals is needed before a conclusive statement on the efficacy of using SIS as nucleus replacement can be made. There is no study in decellularizing allogenic or xenogenic nucleus or annulus for replacement at all. Nevertheless, there is one study evaluating the mechanical properties of decellularized temporomandibular joint disc [73]. Although the immune privileged status of IVD, which is avascular, allows the application of allografts without decellularization, research efforts in decellularizing nucleus and annulus grafts from xenogenic sources for scaffolding should be encouraged because allografts are not always available. Preservation of proteoglycans will, however, be challenging. Recently, there is a surge in number of papers using injectable nucleus replacement, which is based on the fourth scaffolding approach, where cells are encapsulated in self-assembled biomaterials and injected to the disc space before the gel “sets”. Mesenchymal stem cells and disc cells have been encapsulated in thermosensitive hydroxybutyl chitosan gel and cell proliferation and matrix production has been demonstrated [33]. Atellocollagen type II, hyaluronan and aggrecan gels support NP viability [45]. Another study using atellocollagen shows that atellocollagen is better than alginate in supporting NP cells with better water and proteoglycan retention [98]. Nevertheless, most studies on injectable modality using approach 4 are in vitro. Encouraging in vivo studies have been relatively scarce. In a rabbit model, MSCs were loaded in solution atellocollagen [99] and injected to degenerative discs. Disc degeneration is effectively arrested by the treatment, and evidence of atellocollagen supporting cell growth, differentiation and matrix production is demonstrated. In a pig model, MSCs were loaded with hyaluronan derivatives [93] and injected into the nucleotomized discs. Close similarity in disc biconvex structure and viable chondrocytes like cells were reported [93]. Further enhancement in the swelling and mechanical stability of the scaffolds for nucleus or annulus replacement such as crosslinking without compromising cell viability [45] should be encouraged as these physical properties favor the restoration and maintenance of disc height. Evaluation of the mechanical properties of injectable polymers such as hyaluronic acid gel [28] and polyethylene glycol [115] against different engineering parameters should also be encouraged as replacing the mechanical function is equally important as replacing the cellular function in matrix secretion in this stage of degeneration.
At the advanced stage of disc degeneration, structural collapse and eventual loss of disc function in resisting loading set in motion. At this stage, probably only an engineered IVD tissue idealized in the discussion above can do the job. Decellularized IVD allograft is theoretically the best scaffold for late stage replacement. To date, there is no decellularization study in intervertebral disc, but there are several in vivo studies transplanting undecellularized allogenic discs. A few groups have also transplanted cryopreserved allogenic disc grafts in dogs [60, 75]. Nevertheless, dramatic decrease in cellular activity has been reported, indicating that preservation of cellular synthetic and remodeling activities is important for long term viability of the grafts. Furthermore, degenerative changes have been reported at 1 year post-implantation, also suggesting the need to preserve better cellular remodeling capacity. Larger animal model in monkeys [71, 72] showed that fresh frozen allografts can maintain the mechanical properties and some degree of cell metabolism, but severe degeneration has been observed in 2 years time, associating with decreased biochemical contents of the disc. This study also suggests the need to preserve or supplement viable cells with remodeling capacity that is able to maintain long term merits of allografting. Very recently, this group conducted a first allograft disc segment transplantation study in human [96]. The transplanted segments were able to preserve motion and hydration for at least 5 years with partially recovered disc height and improved neurological symptoms, and with no immunoreaction. Nevertheless, mild degenerative changes have been found after years of follow-up, suggesting again that repopulation or supplementation of live cells with matrix remodeling capacity is necessary for long term functionality of allografts. As a result, a promising direction is to develop better graft preservation technologies for maintaining the disc cell remodeling capacity. Alternatively, supplementing stem cells or other cells with the ability to respond to physiological challenges in terms of synthesis and secretion of appropriate ECM into the allograft disc space is also promising. In this regard, searching for injectable carriers to provide an appropriate microenvironment to the encapsulated cells for proper remodeling response toward mechanical stimulation deserves more attention. Apart from using the allograft, building an implantable IVD with multiple tissue components with mechanical properties comparable with the native disc presents another possibility. Activities with this line of research have been scarce, and only a few groups are attempting to build multiple tissue components and to target the interface problems [4, 46]. A few unique considerations should be highlighted as they are the most challenging tasks for tissue engineering. Firstly, IVD is a complex tissue with multiple tissue components. It is unlikely to utilize single biomaterial and single scaffolding approach. Maintaining the differential hydration and mechanical properties of the nucleus and the annulus is important to the maintenance of the disc height and its load-resisting properties. Engineering designs to better retain the water absorbing capacity in the biomaterials used for nucleus replacement and engineering designs to better maintain the enclosure of the swelling nucleus within the confines of the annulus replacement warrant further attention. Secondly, IVD involves multiple tissue interfaces, which are essential for load transfer and distribution between hard and soft tissues [17, 119] and are crucial for maintaining proper disc functions. Chondrocytes seeded between a pre-made bone scaffold and NP cells resulted in the formation of a cartilage-like layer between bone construct and nucleus cells [46] while chondrocytes supplemented with osteogenic differentiation signal led to the formation of a calcified zone between bone and cartilage interface [4]. Both studies demonstrated benefits in mechanical performance, in particular improving the interfacial shear stress. This suggests that engineering the interfaces among different tissue components should deserve special attention and more enthusiastic research efforts.
Disc tissue engineering is by default multidisciplinary, and the scaffolding approach is just one of the disciplines involved. Its success obviously relies on the corroborative efforts from other aspects. For examples, delineation of the etiology of disc degeneration, understanding of the disc nutrition mechanism, cell sourcing, identification of disc specific markers, growth-stimulating and differentiation-stimulating signals, etc. are also important aspects in achieving better functional outcomes of IVD tissue engineering.
Conclusion
Scaffolds in engineered tissues are to mimic the ECM in native tissues, at least partially. Unsurprisingly, their functions should mimic the ECM of the target tissue. Over the past few decades, four major scaffolding approaches, namely implanting cell-seeded pre-made porous scaffolds, implanting cell-seeded decellularized allograft or xenograft ECM, implanting laminated cell sheets with secreted ECM and injecting cell encapsulated self-assembled hydrogels, have been developed. Each approach has its own pros and cons and preferred tissue engineering applications. In planning for tissue engineering for a complex tissue such as IVD, these scaffolding approaches serve as important guidelines and can be used in combinations. Moreover, tissue-specific considerations in relation to the extent of injury, the unique structural functional relationship, multiple tissue composition and interfaces in IVD deserve special attention.
Acknowledgments
This work was supported by grant from AOSpine to Leong and Chan (AOSBRC-07-06), support from NIH (EB003447) to Leong, and grants from Research Grant Council (RGC), Innovation and Technology Commission (ITC) of the Hong Kong Government to Chan.
Conflict of interest statement None of the authors has any potential conflict of interest.
References
- 1.Adams MA, Roughley PJ. What is intervertebral disc degeneration, and what causes it? Spine. 2006;31(18):2151–2161. doi: 10.1097/01.brs.0000231761.73859.2c. [DOI] [PubMed] [Google Scholar]
- 2.Alini M, Li W, Markovic P, Aebi M, Spiro RC, Roughley PJ. The potential and limitations of a cell-seeded collagen/hyaluronan scaffold to engineer an intervertebral disc-like matrix. Spine. 2003;28(5):446–454. doi: 10.1097/00007632-200303010-00007. [DOI] [PubMed] [Google Scholar]
- 3.Alini M, Roughley PJ, Antoniou J, Stoll T, Aebi M. A biological approach to treating disc degeneration: not for today, but maybe for tomorrow. Eur Spine J. 2002;11(Suppl 2):S215–S220. doi: 10.1007/s00586-002-0485-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Allan KS, Pilliar RM, Wang J, Grynpas MD, Kandel RA. Formation of biphasic constructs containing cartilage with a calcified zone interface. Tissue Eng. 2007;13(1):167–177. doi: 10.1089/ten.2006.0081. [DOI] [PubMed] [Google Scholar]
- 5.Allers C, Sierralta WD, Neubauer S, Rivera F, Minguell JJ, Conget PA. Dynamic of distribution of human bone marrow-derived mesenchymal stem cells after transplantation into adult unconditioned mice. Transplantation. 2004;78(4):503–508. doi: 10.1097/01.TP.0000128334.93343.B3. [DOI] [PubMed] [Google Scholar]
- 6.Anderson DG, Risbud MV, Shapiro IM, Vaccaro AR, Albert TJ. Cell-based therapy for disc repair. Spine J. 2005;5(6 Suppl):297S–303S. doi: 10.1016/j.spinee.2005.02.019. [DOI] [PubMed] [Google Scholar]
- 7.Ansaloni L, Cambrini P, Catena F, Di Saverio S, Gagliardi S, Gazzotti F, Hodde JP, Metzger DW, D’Alessandro L, Pinna AD. Immune response to small intestinal submucosa (surgisis) implant in humans: preliminary observations. J Invest Surg. 2007;20(4):237–241. doi: 10.1080/08941930701481296. [DOI] [PubMed] [Google Scholar]
- 8.Ansaloni L, Catena F, Gagliardi S, Gazzotti F, D’Alessandro L, Pinna AD. Hernia repair with porcine small-intestinal submucosa. Hernia. 2007;11(4):321–326. doi: 10.1007/s10029-007-0225-4. [DOI] [PubMed] [Google Scholar]
- 9.Badylak SF. Xenogeneic extracellular matrix as a scaffold for tissue reconstruction. Transpl Immunol. 2004;12(3–4):367–377. doi: 10.1016/j.trim.2003.12.016. [DOI] [PubMed] [Google Scholar]
- 10.Batorsky A, Liao J, Lund AW, Plopper GE, Stegemann JP. Encapsulation of adult human mesenchymal stem cells within collagen-agarose microenvironments. Biotechnol Bioeng. 2005;92(4):492–500. doi: 10.1002/bit.20614. [DOI] [PubMed] [Google Scholar]
- 11.Berger J, Reist M, Mayer JM, Felt O, Peppas NA, Gurny R. Structure and interactions in covalently and ionically crosslinked chitosan hydrogels for biomedical applications. Eur J Pharm Biopharm. 2004;57(1):19–34. doi: 10.1016/S0939-6411(03)00161-9. [DOI] [PubMed] [Google Scholar]
- 12.Bibby SR, Jones DA, Lee RB, Yu J, Urban JPG. The pathophysiology of the intervertebral disc. Joint Bone Spine. 2001;68(6):537–542. doi: 10.1016/S1297-319X(01)00332-3. [DOI] [PubMed] [Google Scholar]
- 13.Bissell DM, Choun MO. The role of extracellular matrix in normal liver. Scand J Gastroenterol Suppl. 1988;151:1–7. doi: 10.3109/00365528809095908. [DOI] [PubMed] [Google Scholar]
- 14.Boccaccini AR, Blaker JJ. Bioactive composite materials for tissue engineering scaffolds. Expert Rev Med Devices. 2005;2(3):303–317. doi: 10.1586/17434440.2.3.303. [DOI] [PubMed] [Google Scholar]
- 15.Borschel GH, Huang YC, Calve S, Arruda EM, Lynch JB, Dow DE, Kuzon WM, Dennis RG, Brown DL. Tissue engineering of recellularized small-diameter vascular grafts. Tissue Eng. 2005;11(5–6):778–786. doi: 10.1089/ten.2005.11.778. [DOI] [PubMed] [Google Scholar]
- 16.Brodie JC, Goldie E, Connel G, Merry J, Grant MH. Osteoblast interactions with calcium phosphate ceramics modified by coating with type I collagen. J Biomed Mater Res A. 2005;73(4):409–421. doi: 10.1002/jbm.a.30279. [DOI] [PubMed] [Google Scholar]
- 17.Broom ND, Poole CA. A functional-morphological study of the tidemark region of articular cartilage maintained in a non-viable physiological condition. J Anat. 1982;135(Pt 1):65–82. [PMC free article] [PubMed] [Google Scholar]
- 18.Chai C, Leong KW. Biomaterials approach to expand and direct differentiation of stem cells. Mol Ther. 2007;15(3):467–480. doi: 10.1038/sj.mt.6300084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chan BP, Chan GCF, Wong HL, Cheung PT, Chan D, Cheah K (2007b) Cell–Matrix Microsphere, Associated Products, Methods for Preparation and Applications. Regular Patent Application No. 60/801,975 (filed on 19 May 2007)
- 20.Chan BP, Hui TY, Chan OC, So KF, Lu W, Cheung KM, Salomatina E, Yaroslavsky A. Photochemical cross-linking for collagen-based scaffolds: a study on optical properties, mechanical properties, stability, and hematocompatibility. Tissue Eng. 2007;13(1):73–85. doi: 10.1089/ten.2006.0004. [DOI] [PubMed] [Google Scholar]
- 21.Chan BP, Hui TY, Yeung CW, Li J, Mo I, Chan GCF. Self-assembled collagen–human mesenchymal stem cell microspheres for regenerative medicine. Biomaterials. 2007;28:4652–4666. doi: 10.1016/j.biomaterials.2007.07.041. [DOI] [PubMed] [Google Scholar]
- 22.Chan BP, So K-F. Photochemical crosslinking improves the physicochemical properties of collagen scaffolds. J Biomed Mater Res A. 2005;75(3):689–701. doi: 10.1002/jbm.a.30469. [DOI] [PubMed] [Google Scholar]
- 23.Chang G, Kim HJ, Kaplan D, Vunjak-Novakovic G, Kandel RA. Porous silk scaffolds can be used for tissue engineering annulus fibrosus. Eur Spine J. 2007;16(11):1848–1857. doi: 10.1007/s00586-007-0364-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chang TM. Hybrid artificial cells: microencapsulation of living cells. ASAIO J. 1992;38(2):128–130. doi: 10.1097/00002480-199204000-00013. [DOI] [PubMed] [Google Scholar]
- 25.Chevalier E, Chulia D, Pouget C, Viana M. Fabrication of porous substrates: a review of processes using pore forming agents in the biomaterial field. J Pharm Sci. 2008;97(3):1135–1154. doi: 10.1002/jps.21059. [DOI] [PubMed] [Google Scholar]
- 26.Chew SY, Mi R, Hoke A, Leong KW. The effect of the alignment of electrospun fibrous scaffolds on Schwann cell maturation. Biomaterials. 2008;29(6):653–661. doi: 10.1016/j.biomaterials.2007.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chew SY, Wen Y, Dzenis Y, Leong KW. The role of electrospinning in the emerging field of nanomedicine. Curr Pharm Des. 2006;12(36):4751–4770. doi: 10.2174/138161206779026326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cloyd JM, Malhotra NR, Weng L, Chen W, Mauck RL, Elliott DM. Material properties in unconfined compression of human nucleus pulposus, injectable hyaluronic acid-based hydrogels and tissue engineering scaffolds. Eur Spine J. 2007;16(11):1892–1898. doi: 10.1007/s00586-007-0443-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Coric D, Mummaneni PV. Nucleus replacement technologies. J Neurosurg Spine. 2008;8(2):115–120. doi: 10.3171/SPI/2008/8/2/115. [DOI] [PubMed] [Google Scholar]
- 30.Crevensten G, Walsh AJ, Ananthakrishnan D, Page P, Wahba GM, Lotz JC, Berven S. Intervertebral disc cell therapy for regeneration: mesenchymal stem cell implantation in rat intervertebral discs. Ann Biomed Eng. 2004;32(3):430–434. doi: 10.1023/B:ABME.0000017545.84833.7c. [DOI] [PubMed] [Google Scholar]
- 31.da Silva RM, Mano JF, Reis RL. Smart thermoresponsive coatings and surfaces for tissue engineering: switching cell-material boundaries. Trends Biotechnol. 2007;25(12):577–583. doi: 10.1016/j.tibtech.2007.08.014. [DOI] [PubMed] [Google Scholar]
- 32.Dang JM, Leong KW. Myogenic induction of aligned mesenchymal stem cell sheets by culture on thermally responsive electrospun nanofibers. Adv Mater. 2007;19(19):2775–2779. doi: 10.1002/adma.200602159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dang JM, Sun DD, Shin-Ya Y, Sieber AN, Kostuik JP, Leong KW. Temperature-responsive hydroxybutyl chitosan for the culture of mesenchymal stem cells and intervertebral disk cells. Biomaterials. 2006;27(3):406–418. doi: 10.1016/j.biomaterials.2005.07.033. [DOI] [PubMed] [Google Scholar]
- 34.Dhariwala B, Hunt E, Boland T. Rapid prototyping of tissue-engineering constructs, using photopolymerizable hydrogels and stereolithography. Tissue Eng. 2004;10(9–10):1316–1322. doi: 10.1089/ten.2004.10.1316. [DOI] [PubMed] [Google Scholar]
- 35.Discher DE, Janmey P, Wang YL. Tissue cells feel and respond to the stiffness of their substrate. Science. 2005;310:1139–1143. doi: 10.1126/science.1116995. [DOI] [PubMed] [Google Scholar]
- 36.Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677–689. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 37.Evans C. Potential biologic therapies for the intervertebral disc. J Bone Joint Surg Am. 2006;88(Suppl 2):95–98. doi: 10.2106/JBJS.E.01328. [DOI] [PubMed] [Google Scholar]
- 38.Fiala R, Vidlar A, Vrtal R, Belej K, Student V. Porcine small intestinal submucosa graft for repair of anterior urethral strictures. Eur Urol. 2007;51(6):1702–1708. doi: 10.1016/j.eururo.2007.01.099. [DOI] [PubMed] [Google Scholar]
- 39.Gazit E. Self-assembled peptide nanostructures: the design of molecular building blocks and their technological utilization. Chem Soc Rev. 2007;36:1263–1269. doi: 10.1039/b605536m. [DOI] [PubMed] [Google Scholar]
- 40.Gilbert TW, Sellaro TL, Badylak SF. Decellularization of tissues and organs. Biomaterials. 2006;27(19):3675–3683. doi: 10.1016/j.biomaterials.2006.02.014. [DOI] [PubMed] [Google Scholar]
- 41.Gray DW. An overview of the immune system with specific reference to membrane encapsulation and islet transplantation. Ann N Y Acad Sci. 2001;944:226–239. doi: 10.1111/j.1749-6632.2001.tb03835.x. [DOI] [PubMed] [Google Scholar]
- 42.Grohn P, Klock G, Zimmermann U. Collagen-coated Ba(2+)-alginate microcarriers for the culture of anchorage-dependent mammalian cells. Biotechniques. 1997;22(5):970–975. doi: 10.2144/97225rr06. [DOI] [PubMed] [Google Scholar]
- 43.Gruber HE, Hanley EN., Jr Biologic strategies for the therapy of intervertebral disc degeneration. Expert Opin Biol Ther. 2003;3(8):1209–1214. doi: 10.1517/14712598.3.8.1209. [DOI] [PubMed] [Google Scholar]
- 44.Hall S. Axonal regeneration through acellular muscle grafts. J Anat. 1997;190(1):57–71. doi: 10.1046/j.1469-7580.1997.19010057.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Halloran DO, Grad S, Stoddart M, Dockery P, Alini M, Pandit AS. An injectable cross-linked scaffold for nucleus pulposus regeneration. Biomaterials. 2008;29(4):438–447. doi: 10.1016/j.biomaterials.2007.10.009. [DOI] [PubMed] [Google Scholar]
- 46.Hamilton DJ, Séguin CA, Wang J, Pilliar RM, Kandel RA. Bioengineering of skeletal tissues team: formation of a nucleus pulposus-cartilage endplate construct in vitro. Biomaterials. 2006;27(3):397–405. doi: 10.1016/j.biomaterials.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 47.Hersel U, Dahmen C, Kessler H. RGD modified polymers: biomaterials for stimulated cell adhesion and beyond. Biomaterials. 2003;24(24):4385–4415. doi: 10.1016/S0142-9612(03)00343-0. [DOI] [PubMed] [Google Scholar]
- 48.Ho G, Leung VY, Cheung KM, Chan D. Effect of severity of intervertebral disc injury on mesenchymal stem cell-based regeneration. Connect Tissue Res. 2008;49(1):15–21. doi: 10.1080/03008200701818595. [DOI] [PubMed] [Google Scholar]
- 49.Hodde J. Extracellular matrix as a bioactive material for soft tissue reconstruction. ANZ J Surg. 2006;76(12):1096–1100. doi: 10.1111/j.1445-2197.2006.03948.x. [DOI] [PubMed] [Google Scholar]
- 50.Hollister SJ. Porous scaffold design for tissue engineering. Nat Mater. 2005;4(7):518–524. doi: 10.1038/nmat1421. [DOI] [PubMed] [Google Scholar]
- 51.Hubbell JA. Biomaterials in tissue engineering. Biotechnology (N Y) 1995;13(6):565–576. doi: 10.1038/nbt0695-565. [DOI] [PubMed] [Google Scholar]
- 52.Hui TY, Cheung KMC, Cheung WL, Chan D, Chan BP. In vitro chondrogenic differentiation of human mesenchymal stem cells in collagen microspheres: influence of cell seeding density and collagen concentration. Biomaterials. 2008;29:3201–3212. doi: 10.1016/j.biomaterials.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 53.Humes HD. Stem cells: the next therapeutic frontier. Trans Am Clin Climatol Assoc. 2005;116:167–183. [PMC free article] [PubMed] [Google Scholar]
- 54.Hutmacher DW, Sittinger M, Risbud MV. Scaffold-based tissue engineering: rationale for computer-aided design and solid free-form fabrication systems. Trends Biotechnol. 2004;22(7):354–362. doi: 10.1016/j.tibtech.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 55.Ifkovits JL, Burdick JA. Review: photopolymerizable and degradable biomaterials for tissue engineering applications. Tissue Eng. 2007;13(10):2369–2385. doi: 10.1089/ten.2007.0093. [DOI] [PubMed] [Google Scholar]
- 56.Ingram JH, Korossis S, Howling G, Fisher J, Ingham E. The use of ultrasonication to aid recellularization of acellular natural tissue scaffolds for use in anterior cruciate ligament reconstruction. Tissue Eng. 2007;13(7):1561–1572. doi: 10.1089/ten.2006.0362. [DOI] [PubMed] [Google Scholar]
- 57.Isenberg BC, Tsuda Y, Williams C, Shimizu T, Yamato M, Okano T, Wong JY. A thermoresponsive, microtextured substrate for cell sheet engineering with defined structural organization. Biomaterials. 2008;29(17):2565–2572. doi: 10.1016/j.biomaterials.2008.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Iwata H, Amemiya H, Hayashi R, Fujii S, Akutsu T. The use of photocrosslinkable polyvinyl alcohol in the immunoisolation of pancreatic islets. Transplant Proc. 1990;22(2):797–799. [PubMed] [Google Scholar]
- 59.Karp JM, Langer R. Development and therapeutic applications of advanced biomaterials. Curr Opin Biotechnol. 2007;18(5):454–459. doi: 10.1016/j.copbio.2007.09.008. [DOI] [PubMed] [Google Scholar]
- 60.Katsuura A, Hukuda S. Experimental study of intervertebral disc allografting in the dog. Spine. 1994;19(21):2426–2432. doi: 10.1097/00007632-199411000-00011. [DOI] [PubMed] [Google Scholar]
- 61.Kim BS, Baez CE, Atala A. Biomaterials for tissue engineering. World J Urol. 2000;18(1):2–9. doi: 10.1007/s003450050002. [DOI] [PubMed] [Google Scholar]
- 62.Knight RL, Wilcox HE, Korossis SA, Fisher J, Ingham E. The use of acellular matrices for the tissue engineering of cardiac valves. Proc Inst Mech Eng [H] 2008;222(1):129–143. doi: 10.1243/09544119JEIM230. [DOI] [PubMed] [Google Scholar]
- 63.Kurella A, Dahotre NB. Review paper: surface modification for bioimplants: the role of laser surface engineering. J Biomater Appl. 2005;20(1):5–50. doi: 10.1177/0885328205052974. [DOI] [PubMed] [Google Scholar]
- 64.Langer R, Tirrell DA. Designing materials for biology and medicine. Nature. 2004;428(6982):487–492. doi: 10.1038/nature02388. [DOI] [PubMed] [Google Scholar]
- 65.Langer R, Vacanti JP. Tissue engineering. Science. 1993;260(5110):920–926. doi: 10.1126/science.8493529. [DOI] [PubMed] [Google Scholar]
- 66.Lanza RP, Hayes JL, Chick WL. Encapsulated cell technology. Nat Biotechnol. 1996;14(9):1107–1111. doi: 10.1038/nbt0996-1107. [DOI] [PubMed] [Google Scholar]
- 67.Le Visage C, Kim SW, Tateno K, Sieber AN, Kostuik JP, Leong KW. Interaction of human mesenchymal stem cells with disc cells: changes in extracellular matrix biosynthesis. Spine. 2006;31(18):2036–2042. doi: 10.1097/01.brs.0000231442.05245.87. [DOI] [PubMed] [Google Scholar]
- 68.Le Visage C, Yang SH, Kadakia L, Sieber AN, Kostuik JP, Leong KW. Small intestinal submucosa as a potential bioscaffold for intervertebral disc regeneration. Spine. 2006;31(21):2423–2430. doi: 10.1097/01.brs.0000238684.04792.eb. [DOI] [PubMed] [Google Scholar]
- 69.Le Visage K, Dang JM, Chan BP, Serhan H, Sieber AN, Kostuik JP, Leong KW (2008) Biomaterials development for disc degeneration: a pilot study of small intestinal submucosa for nucleus pulposus augmentation in a non-human primate model. World Forum for Spine Research—the intervertebral disc. First Japanese Meeting, 23–26 January 2008, The Westin Miyako Kyoto, Kyoto, Japan
- 70.Leone G, Torricelli P, Chiumiento A, Facchini A, Barbucci R. Amidic alginate hydrogel for nucleus pulposus replacement. J Biomed Mater Res A. 2008;84(2):391–401. doi: 10.1002/jbm.a.31334. [DOI] [PubMed] [Google Scholar]
- 71.Luk KD, Ruan DK, Chow DH, Leong JC. Intervertebral disc autografting in a bipedal animal model. Clin Orthop Relat Res. 1997;337:13–26. doi: 10.1097/00003086-199704000-00003. [DOI] [PubMed] [Google Scholar]
- 72.Luk KD, Ruan DK, Lu DS, Fei ZQ. Fresh frozen intervertebral disc allografting in a bipedal animal model. Spine. 2003;28(9):864–869. doi: 10.1097/00007632-200305010-00005. [DOI] [PubMed] [Google Scholar]
- 73.Lumpkins SB, Pierre N, McFetridge PS. A mechanical evaluation of three decellularization methods in the design of a xenogeneic scaffold for tissue engineering the temporomandibular joint disc. Acta Biomater. 2008;4(4):808–816. doi: 10.1016/j.actbio.2008.01.016. [DOI] [PubMed] [Google Scholar]
- 74.Masuda K, An HS. Prevention of disc degeneration with growth factors. Eur Spine J. 2006;15(Suppl 3):S422–S432. doi: 10.1007/s00586-006-0149-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Matsuzaki H, Wakabayashi K, Ishihara K, Ishikawa H, Ohkawa A. Allografting intervertebral discs in dogs: a possible clinical application. Spine. 1996;21(2):178–183. doi: 10.1097/00007632-199601150-00004. [DOI] [PubMed] [Google Scholar]
- 76.Mizuno H, Roy AK, Vacanti CA, Kojima K, Ueda M, Bonassar LJ. Tissue-engineered composites of anulus fibrosus and nucleus pulposus for intervertebral disc replacement. Spine. 2004;29(12):1290–1297. doi: 10.1097/01.BRS.0000128264.46510.27. [DOI] [PubMed] [Google Scholar]
- 77.Mligiliche N, Endo K, Okamoto K, Fujimoto E, Ide C. Extracellular matrix of human amnion manufactured into tubes as conduits for peripheral nerve regeneration. J Biomed Mater Res. 2002;63(5):591–600. doi: 10.1002/jbm.10349. [DOI] [PubMed] [Google Scholar]
- 78.Morra M, Cassinelli C. Biomaterials surface characterization and modification. Int J Artif Organs. 2006;29(9):824–833. doi: 10.1177/039139880602900903. [DOI] [PubMed] [Google Scholar]
- 79.Muschler GF, Nakamoto C, Griffith LG. Engineering principles of clinical cell-based tissue engineering. J Bone Joint Surg Am. 2004;86-A(7):1541–1558. doi: 10.2106/00004623-200407000-00029. [DOI] [PubMed] [Google Scholar]
- 80.Nerurkar NL, Elliott DM, Mauck RL. Mechanics of oriented electrospun nanofibrous scaffolds for annulus fibrosus tissue engineering. J Orthop Res. 2007;25(8):1018–1028. doi: 10.1002/jor.20384. [DOI] [PubMed] [Google Scholar]
- 81.Nguyen KT, West JL. Photopolymerizable hydrogels for tissue engineering applications. Biomaterials. 2002;23(22):4307–4314. doi: 10.1016/S0142-9612(02)00175-8. [DOI] [PubMed] [Google Scholar]
- 82.Nishida K, Yamato M, Hayashida Y, Watanabe K, Yamamoto K, Adachi E, Nagai S, Kikuchi A, Maeda N, Watanabe H, Okano T, Tano Y. Corneal reconstruction with tissue-engineered cell sheets composed of autologous oral mucosal epithelium. N Engl J Med. 2004;351(12):1187–1196. doi: 10.1056/NEJMoa040455. [DOI] [PubMed] [Google Scholar]
- 83.Nuttelman CR, Tripodi MC, Anseth KS. Synthetic hydrogel niches that promote hMSC viability. Matrix Biol. 2005;24(3):208–218. doi: 10.1016/j.matbio.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 84.Okano T, Yamada N, Okuhara M, Sakai H, Sakurai Y. Mechanism of cell detachment from temperature-modulated, hydrophilic–hydrophobic polymer surfaces. Biomaterials. 1995;16(4):297–303. doi: 10.1016/0142-9612(95)93257-E. [DOI] [PubMed] [Google Scholar]
- 85.Okano T, Yamada N, Sakai H, Sakurai Y. A novel recovery system for cultured cells using plasma-treated polystyrene dishes grafted with poly(N-isopropylacrylamide) J Biomed Mater Res. 1993;27(10):1243–1251. doi: 10.1002/jbm.820271005. [DOI] [PubMed] [Google Scholar]
- 86.Orive G, Hernández RM, Gascón AR, Calafiore R, Chang TM, Vos P, Hortelano G, Hunkeler D, Lacík I, Shapiro AM, Pedraz JL. Cell encapsulation: promise and progress. Nat Med. 2003;9(1):104–107. doi: 10.1038/nm0103-104. [DOI] [PubMed] [Google Scholar]
- 87.Orive G, Hernández RM, Rodríguez Gascón A, Calafiore R, Chang TM, Vos P, Hortelano G, Hunkeler D, Lacík I, Pedraz JL. History, challenges and perspectives of cell microencapsulation. Trends Biotechnol. 2004;22(2):87–92. doi: 10.1016/j.tibtech.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 88.Paesold G, Nerlich AG, Boos N. Biological treatment strategies for disc degeneration: potentials and shortcomings. Eur Spine J. 2007;16(4):447–468. doi: 10.1007/s00586-006-0220-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Pham QP, Sharma U, Mikos AG. Electrospinning of polymeric nanofibers for tissue engineering applications: a review. Tissue Eng. 2006;12(5):1197–1211. doi: 10.1089/ten.2006.12.1197. [DOI] [PubMed] [Google Scholar]
- 90.Piez KA. History of extracellular matrix: a personal view. Matrix Biol. 1997;16(3):85–92. doi: 10.1016/S0945-053X(97)90037-8. [DOI] [PubMed] [Google Scholar]
- 91.Poole AR, Kojima T, Yasuda T, Mwale F, Kobayashi M, Laverty S. Composition and structure of articular cartilage: a template for tissue repair (review) Clin Orthop Relat Res. 2001;391(Supp):S26–S33. doi: 10.1097/00003086-200110001-00004. [DOI] [PubMed] [Google Scholar]
- 92.Ratner BD, Bryant SJ. Biomaterials: where we have been and where we are going. Annu Rev Biomed Eng. 2004;6:41–75. doi: 10.1146/annurev.bioeng.6.040803.140027. [DOI] [PubMed] [Google Scholar]
- 93.Revell PA, Damien E, Di Silvio L, Gurav N, Longinotti C, Ambrosio L. Tissue engineered intervertebral disc repair in the pig using injectable polymers. J Mater Sci Mater Med. 2007;18(2):303–308. doi: 10.1007/s10856-006-0693-6. [DOI] [PubMed] [Google Scholar]
- 94.Richardson SM, Curran JM, Chen R, Vaughan-Thomas A, Hunt JA, Freemont AJ, Hoyland JA. The differentiation of bone marrow mesenchymal stem cells into chondrocyte-like cells on poly-L-lactic acid (PLLA) scaffolds. Biomaterials. 2006;27(22):4069–4078. doi: 10.1016/j.biomaterials.2006.03.017. [DOI] [PubMed] [Google Scholar]
- 95.Robert L. Matrix biology: past, present and future. Pathol Biol (Paris) 2001;49(4):279–283. doi: 10.1016/s0369-8114(01)00141-9. [DOI] [PubMed] [Google Scholar]
- 96.Ruan D, He Q, Ding Y, Hou L, Li J, Luk KD. Intervertebral disc transplantation in the treatment of degenerative spine disease: a preliminary study. Lancet. 2007;369(9566):993–999. doi: 10.1016/S0140-6736(07)60496-6. [DOI] [PubMed] [Google Scholar]
- 97.Saad L, Spector M. Effects of collagen type on the behavior of adult canine annulus fibrosus cells in collagen-glycosaminoglycan scaffolds. J Biomed Mater Res A. 2004;71(2):233–241. doi: 10.1002/jbm.a.30150. [DOI] [PubMed] [Google Scholar]
- 98.Sakai D, Mochida J, Iwashina T, Watanabe T, Suyama K, Ando K, Hotta T. Atelocollagen for culture of human nucleus pulposus cells forming nucleus pulposus-like tissue in vitro: influence on the proliferation and proteoglycan production of HNPSV-1 cells. Biomaterials. 2006;27(3):346–353. doi: 10.1016/j.biomaterials.2005.06.040. [DOI] [PubMed] [Google Scholar]
- 99.Sakai D, Mochida J, Yamamoto Y, Nomura T, Okuma M, Nishimura K, Nakai T, Ando K, Hotta T. Transplantation of mesenchymal stem cells embedded in Atelocollagen gel to the intervertebral disc: a potential therapeutic model for disc degeneration. Biomaterials. 2003;24(20):3531–3541. doi: 10.1016/S0142-9612(03)00222-9. [DOI] [PubMed] [Google Scholar]
- 100.Sano A, Maeda M, Nagahara S, Ochiya T, Honma K, Itoh H, Miyata T, Fujioka K. Atelocollagen for protein and gene delivery. Adv Drug Deliv Rev. 2003;55(12):1651–1677. doi: 10.1016/j.addr.2003.08.005. [DOI] [PubMed] [Google Scholar]
- 101.Schmidt CE, Baier JM. Acellular vascular tissues: natural biomaterials for tissue repair and tissue engineering. Biomaterials. 2000;21(22):2215–2231. doi: 10.1016/S0142-9612(00)00148-4. [DOI] [PubMed] [Google Scholar]
- 102.Schönherr E, Hausser HJ. Extracellular matrix and cytokines: a functional unit. Dev Immunol. 2000;7(2–4):89–101. doi: 10.1155/2000/31748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Sebastine IM, Williams DJ. Current developments in tissue engineering of nucleus pulposus for the treatment of intervertebral disc degeneration. Conf Proc IEEE Eng Med Biol Soc. 2007;2007:6401–6406. doi: 10.1109/IEMBS.2007.4353821. [DOI] [PubMed] [Google Scholar]
- 104.Sekiya S, Shimizu T, Yamato M, Kikuchi A, Okano T. Bioengineered cardiac cell sheet grafts have intrinsic angiogenic potential. Biochem Biophys Res Commun. 2006;341(2):573–582. doi: 10.1016/j.bbrc.2005.12.217. [DOI] [PubMed] [Google Scholar]
- 105.Shimizu T, Sekine H, Yang J, Isoi Y, Yamato M, Kikuchi A, Kobayashi E, Okano T. Polysurgery of cell sheet grafts overcomes diffusion limits to produce thick, vascularized myocardial tissues. FASEB J. 2006;20(6):708–710. doi: 10.1096/fj.05-4715fje. [DOI] [PubMed] [Google Scholar]
- 106.Shimizu T, Yamato M, Kikuchi A, Okano T. Cell sheet engineering for myocardial tissue reconstruction. Biomaterials. 2003;24(13):2309–2316. doi: 10.1016/S0142-9612(03)00110-8. [DOI] [PubMed] [Google Scholar]
- 107.Sill TJ, Recum HA. Electrospinning: applications in drug delivery and tissue engineering. Biomaterials. 2008;29(13):1989–2006. doi: 10.1016/j.biomaterials.2008.01.011. [DOI] [PubMed] [Google Scholar]
- 108.Sobajima S, Vadala G, Shimer A, Kim JS, Gilbertson LG, Kang JD (2008) Feasibility of a stem cell therapy for intervertebral disc degeneration. Spine J December (in press) [DOI] [PubMed]
- 109.Spector M. Biomaterials-based tissue engineering and regenerative medicine solutions to musculoskeletal problems. Swiss Med Wkly. 2006;136(19–20):293–301. doi: 10.4414/smw.2006.11310. [DOI] [PubMed] [Google Scholar]
- 110.Takezawa T, Mori Y, Yoshizato K. Cell culture on a thermo-responsive polymer surface. Biotechnology (N Y) 1990;8(9):854–856. doi: 10.1038/nbt0990-854. [DOI] [PubMed] [Google Scholar]
- 111.Tsuda Y, Shimizu T, Yamato M, Kikuchi A, Sasagawa T, Sekiya S, Kobayashi J, Chen G, Okano T. Cellular control of tissue architectures using a three-dimensional tissue fabrication technique. Biomaterials. 2007;28(33):4939–4946. doi: 10.1016/j.biomaterials.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 112.Uitto J, Olsen DR, Fazio MJ. Extracellular matrix of the skin: 50 years of progress. J Invest Dermatol. 1989;92(4 Suppl):61S–77S. doi: 10.1111/1523-1747.ep13075039. [DOI] [PubMed] [Google Scholar]
- 113.Uludag H, Vos P, Tresco PA. Technology of mammalian cell encapsulation. Adv Drug Deliv Rev. 2000;42(1–2):29–64. doi: 10.1016/S0169-409X(00)00053-3. [DOI] [PubMed] [Google Scholar]
- 114.Vacanti JP, Langer R. Tissue engineering: the design and fabrication of living replacement devices for surgical reconstruction and transplantation. Lancet. 1999;354(Suppl 1):SI32–SI34. doi: 10.1016/s0140-6736(99)90247-7. [DOI] [PubMed] [Google Scholar]
- 115.Vernengo J, Fussell GW, Smith NG, Lowman AM. Evaluation of novel injectable hydrogels for nucleus pulposus replacement. J Biomed Mater Res B Appl Biomater. 2008;84(1):64–69. doi: 10.1002/jbm.b.30844. [DOI] [PubMed] [Google Scholar]
- 116.Walker MH, Anderson DG. Molecular basis of intervertebral disc degeneration. Spine J. 2004;4(6 Suppl):158S–166S. doi: 10.1016/j.spinee.2004.07.010. [DOI] [PubMed] [Google Scholar]
- 117.Wan AC, Yim EK, Liao IC, Le Visage C, Leong KW. Encapsulation of biologics in self-assembled fibers as biostructural units for tissue engineering. J Biomed Mater Res A. 2004;71(4):586–595. doi: 10.1002/jbm.a.30158. [DOI] [PubMed] [Google Scholar]
- 118.Wan Y, Feng G, Shen FH, Laurencin CT, Li X. Biphasic scaffold for annulus fibrosus tissue regeneration. Biomaterials. 2008;29(6):643–652. doi: 10.1016/j.biomaterials.2007.10.031. [DOI] [PubMed] [Google Scholar]
- 119.Wang IN, Lu HH. Role of cell–cell interactions on the regeneration of soft tissue-to-bone interface. Conf Proc IEEE Eng Med Biol Soc. 2006;1:783–786. doi: 10.1109/IEMBS.2006.4397518. [DOI] [PubMed] [Google Scholar]
- 120.Wilda H, Gough JE. In vitro studies of annulus fibrosus disc cell attachment, differentiation and matrix production on PDLLA/45S5 Bioglass composite films. Biomaterials. 2006;27(30):5220–5229. doi: 10.1016/j.biomaterials.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 121.Wilke HJ, Heuer F, Neidlinger-Wilke C, Claes L. Is a collagen scaffold for a tissue engineered nucleus replacement capable of restoring disc height and stability in an animal model? Eur Spine J. 2006;15(Suppl 3):S433–S438. doi: 10.1007/s00586-006-0177-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Wong HL, Wang MX, Cheung PT, Yao KM, Chan BP. A 3D collagen microsphere culture system for GDNF-secreting HEK293 cells with enhanced protein productivity. Biomaterials. 2007;28:5369–5380. doi: 10.1016/j.biomaterials.2007.08.016. [DOI] [PubMed] [Google Scholar]
- 123.Yang J, Yamato M, Shimizu T, Sekine H, Ohashi K, Kanzaki M, Ohki T, Nishida K, Okano T. Reconstruction of functional tissues with cell sheet engineering. Biomaterials. 2007;28(34):5033–5043. doi: 10.1016/j.biomaterials.2007.07.052. [DOI] [PubMed] [Google Scholar]
- 124.Yang S, Leong KF, Du Z, Chua CK. The design of scaffolds for use in tissue engineering: Part II. Rapid prototyping techniques. Tissue Eng. 2002;8(1):1–11. doi: 10.1089/107632702753503009. [DOI] [PubMed] [Google Scholar]
- 125.Yang S, Leong KF, Du Z, Chua CK. The design of scaffolds for use in tissue engineering: Part I. Traditional factors. Tissue Eng. 2001;7(6):679–689. doi: 10.1089/107632701753337645. [DOI] [PubMed] [Google Scholar]
- 126.Yannas IV. Natural materials. In: Ratner BD, Hoffman AS, Schoen FJ, Lemons JE, editors. Biomaterials Sciences: an introduction to materials in medicine. New York: Academic Press; 1996. pp. 84–93. [Google Scholar]
- 127.Yim EK, Reano RM, Pang SW, Yee AF, Chen CS, Leong KW. Nanopattern-induced changes in morphology and motility of smooth muscle cells. Biomaterials. 2005;26(26):5405–5413. doi: 10.1016/j.biomaterials.2005.01.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Zhang S. Fabrication of novel biomaterials through molecular self-assembly. Nat Biotechnol. 2003;21(10):1171–1178. doi: 10.1038/nbt874. [DOI] [PubMed] [Google Scholar]
- 129.Zheng MH, Chen J, Kirilak Y, Willers C, Xu J, Wood D. Porcine small intestine submucosa (SIS) is not an acellular collagenous matrix and contains porcine DNA: possible implications in human implantation. J Biomed Mater Res B Appl Biomater. 2005;73(1):61–67. doi: 10.1002/jbm.b.30170. [DOI] [PubMed] [Google Scholar]
- 130.Zielinski BA, Aebischer P. Chitosan as a matrix for mammalian cell encapsulation. Biomaterials. 1994;15(13):1049–1056. doi: 10.1016/0142-9612(94)90090-6. [DOI] [PubMed] [Google Scholar]
- 131.Zimmermann H, Hillgartner M, Manz B, Feilen P, Brunnenmeier F, Leinfelder U, Weber M, Cramer H, Schneider S, Hendrich C, Volke F, Zimmermann U. Fabrication of homogeneously cross-linked, functional alginate microcapsules validated by NMR-, CLSM- and AFM-imaging. Biomaterials. 2003;24(12):2083–2096. doi: 10.1016/S0142-9612(02)00639-7. [DOI] [PubMed] [Google Scholar]