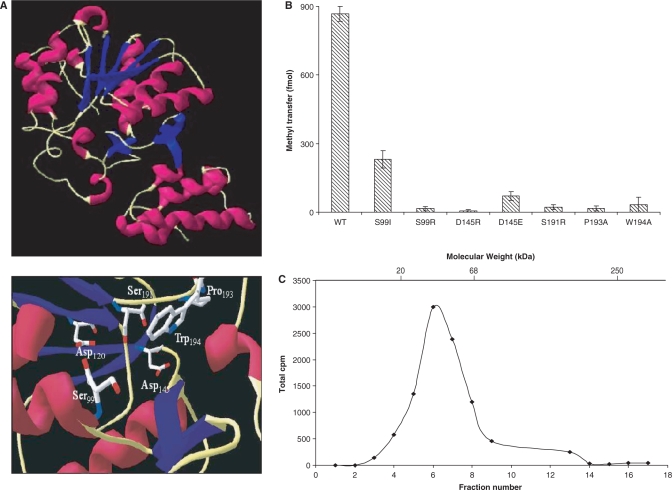
Figure 3.
Structure–function analysis of recombinant TvTgs. (A) A ribbon representation of the predicted TvTgs structure (top) and predicted catalytic and donor sites (bottom) indicated by homology modelling are shown. Conserved amino acids known to be indispensable for S. cerevisiae TvTgs activity (22,24) are presented (bottom). (B) Methyltransferase activity was measured using saturating amounts of m7GDP (10 mM) as substrate and equivalent amounts of wild-type (WT) and mutant TvTgs proteins as indicated. (C) Zonal velocity sedimentation analysis of TvTgs. Recombinant TvTgs and internal molecular weight standards (20-kDa soybean trypsin inhibitor, 66-kDa bovine serum albumin and 250-kDa catalase) were fractionated on a sucrose gradient. Aliquots of each fraction were then tested for methyltransferase activity, using [methyl-3H]AdoMet as the methyl donor. The peaks for separation of the protein standards, as determined using SDS–PAGE, are indicated at the top.