Abstract
The development of a small-animal model to test the protective efficacy and immunogenicity of a vaccine strain against shigellosis would greatly facilitate the evaluation of potential vaccine candidates. In guinea pigs, the ability of shigellae to invade and multiply within the corneal epithelium, causing keratoconjunctivitis, closely mimics the invasion process in the intestinal epithelium (B. Sereny, Acta Microbiol. Acad. Sci. Hung. 4:367-376, 1957). The serum response of animals recovering from a Shigella keratoconjunctival infection was determined and found to be consistent with that shown by convalescent humans and primates. This model was used to test the efficacy of two vaccine candidates, and the immune response of the guinea pigs to the vaccine strains was examined. Both vaccine strains demonstrated significant protection against challenge by homologous virulent Shigella strains, and the results were comparable with results obtained in trials with monkeys. The guinea pig model also provides a rapid and inexpensive means of evaluating different immunization regimens as well as of testing other variables such as length of protection against disease.
Full text
PDF

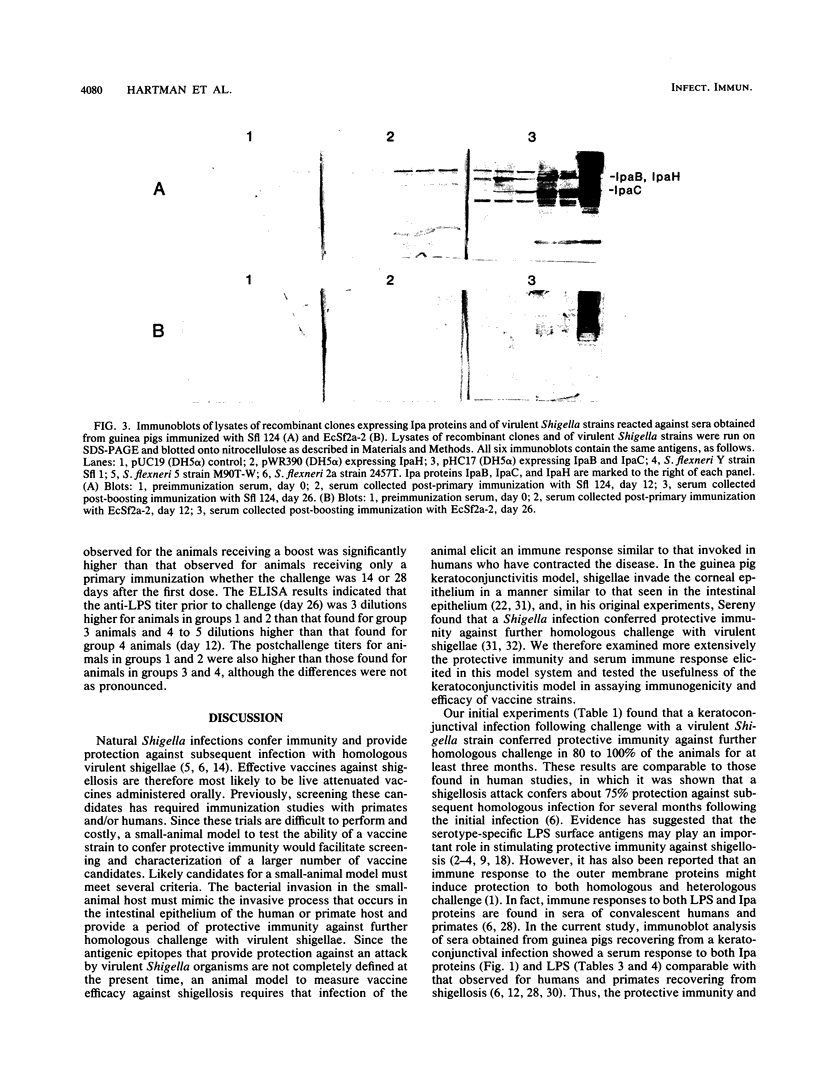
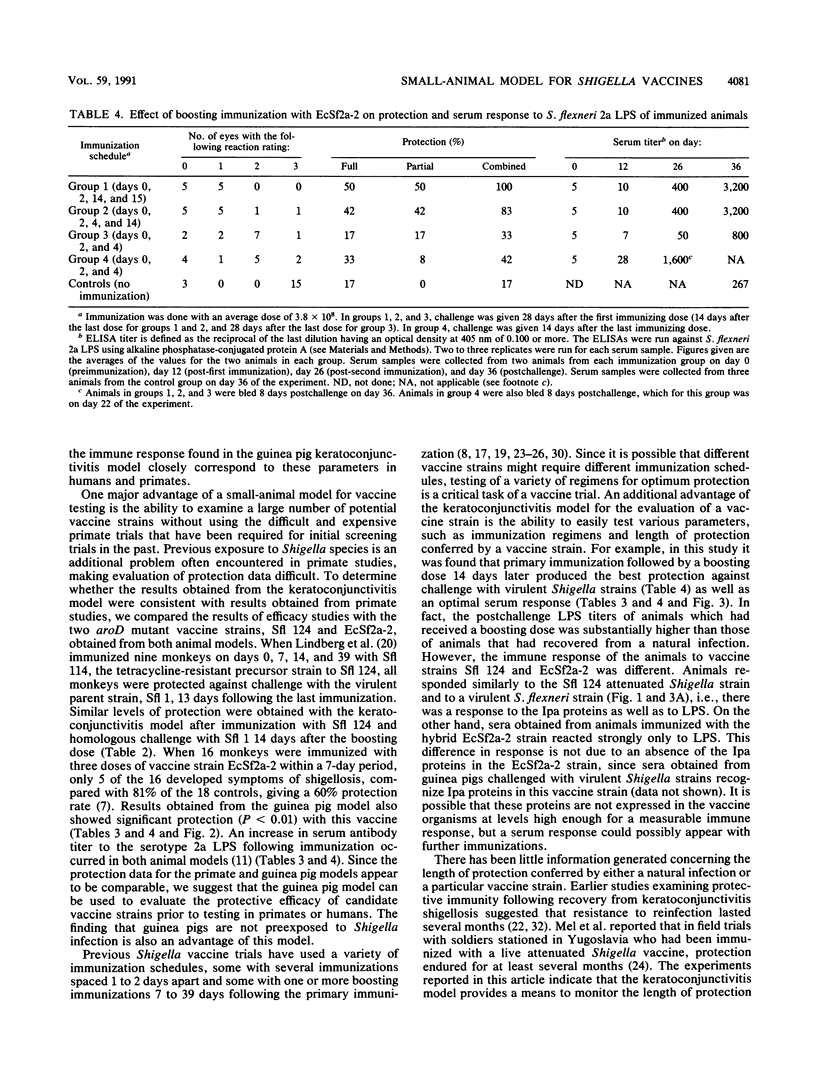


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adamus G., Mulczyk M., Witkowska D., Romanowska E. Protection against keratoconjunctivitis shigellosa induced by immunization with outer membrane proteins of Shigella spp. Infect Immun. 1980 Nov;30(2):321–324. doi: 10.1128/iai.30.2.321-324.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black R. E., Levine M. M., Clements M. L., Losonsky G., Herrington D., Berman S., Formal S. B. Prevention of shigellosis by a Salmonella typhi-Shigella sonnei bivalent vaccine. J Infect Dis. 1987 Jun;155(6):1260–1265. doi: 10.1093/infdis/155.6.1260. [DOI] [PubMed] [Google Scholar]
- Cohen D., Green M. S., Block C., Rouach T., Ofek I. Serum antibodies to lipopolysaccharide and natural immunity to shigellosis in an Israeli military population. J Infect Dis. 1988 May;157(5):1068–1071. doi: 10.1093/infdis/157.5.1068. [DOI] [PubMed] [Google Scholar]
- Cohen D., Green M. S., Block C., Slepon R., Ofek I. Prospective study of the association between serum antibodies to lipopolysaccharide O antigen and the attack rate of shigellosis. J Clin Microbiol. 1991 Feb;29(2):386–389. doi: 10.1128/jcm.29.2.386-389.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinari G., Hale T. L., Austin S. W., Formal S. B. Local and systemic antibody responses to Shigella infection in rhesus monkeys. J Infect Dis. 1987 May;155(5):1065–1069. doi: 10.1093/infdis/155.5.1065. [DOI] [PubMed] [Google Scholar]
- DuPont H. L., Hornick R. B., Snyder M. J., Libonati J. P., Formal S. B., Gangarosa E. J. Immunity in shigellosis. II. Protection induced by oral live vaccine or primary infection. J Infect Dis. 1972 Jan;125(1):12–16. doi: 10.1093/infdis/125.1.12. [DOI] [PubMed] [Google Scholar]
- Formal S. B., Hale T. L., Kapfer C., Cogan J. P., Snoy P. J., Chung R., Wingfield M. E., Elisberg B. L., Baron L. S. Oral vaccination of monkeys with an invasive Escherichia coli K-12 hybrid expressing Shigella flexneri 2a somatic antigen. Infect Immun. 1984 Nov;46(2):465–469. doi: 10.1128/iai.46.2.465-469.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Formal S. B., Maenza R. M., Austin S., LaBrec E. H. Failure of parenteral vaccines to protect monkeys against experimental shigellosis. Proc Soc Exp Biol Med. 1967 Jun;125(2):347–349. doi: 10.3181/00379727-125-32087. [DOI] [PubMed] [Google Scholar]
- HIGGINS A. R., FLOYD T. M., KADER M. A. Studies in shigellosis. III. A controlled evaluation of a monovalent Shigella vaccine in a highly endemic environment. Am J Trop Med Hyg. 1955 Mar;4(2):281–288. [PubMed] [Google Scholar]
- Hale T. L. Hybrid vaccines using Escherichia coli as an antigen carrier. Res Microbiol. 1990 Sep-Oct;141(7-8):913–919. doi: 10.1016/0923-2508(90)90130-i. [DOI] [PubMed] [Google Scholar]
- Hartman A. B., Venkatesan M., Oaks E. V., Buysse J. M. Sequence and molecular characterization of a multicopy invasion plasmid antigen gene, ipaH, of Shigella flexneri. J Bacteriol. 1990 Apr;172(4):1905–1915. doi: 10.1128/jb.172.4.1905-1915.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrington D. A., Van de Verg L., Formal S. B., Hale T. L., Tall B. D., Cryz S. J., Tramont E. C., Levine M. M. Studies in volunteers to evaluate candidate Shigella vaccines: further experience with a bivalent Salmonella typhi-Shigella sonnei vaccine and protection conferred by previous Shigella sonnei disease. Vaccine. 1990 Aug;8(4):353–357. doi: 10.1016/0264-410x(90)90094-3. [DOI] [PubMed] [Google Scholar]
- Kowalewska D., Mulczyk M. Immunity to keratoconjunctivitis shigellosa in rabbits after oral immunization with Shigella sonnei free endotoxin. Acta Microbiol Acad Sci Hung. 1974;21(1-2):99–102. [PubMed] [Google Scholar]
- Levine M. M., Kaper J. B., Black R. E., Clements M. L. New knowledge on pathogenesis of bacterial enteric infections as applied to vaccine development. Microbiol Rev. 1983 Dec;47(4):510–550. doi: 10.1128/mr.47.4.510-550.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindberg A. A., Karnell A., Pál T., Sweiha H., Hultenby K., Stocker B. A. Construction of an auxotrophic Shigella flexneri strain for use as a live vaccine. Microb Pathog. 1990 Jun;8(6):433–440. doi: 10.1016/0882-4010(90)90030-t. [DOI] [PubMed] [Google Scholar]
- Lindberg A. A., Kärnell A., Stocker B. A., Katakura S., Sweiha H., Reinholt F. P. Development of an auxotrophic oral live Shigella flexneri vaccine. Vaccine. 1988 Apr;6(2):146–150. doi: 10.1016/s0264-410x(88)80018-5. [DOI] [PubMed] [Google Scholar]
- Linde K., Dentchev V., Bondarenko V., Marinova S., Randhagen B., Bratoyeva M., Tsvetanov Y., Romanova Y. Live Shigella flexneri 2a and Shigella sonnei I vaccine candidate strains with two attenuating markers. I. Construction of vaccine candidate strains with retained invasiveness but reduced intracellular multiplication. Vaccine. 1990 Feb;8(1):25–29. doi: 10.1016/0264-410x(90)90173-j. [DOI] [PubMed] [Google Scholar]
- MACKEL D. C., LANGLEY L. F., VENICE L. A. The use of the guinea-pig conjunctivae as an experimental model for the study of virulence of Shigella organisms. Am J Hyg. 1961 Mar;73:219–223. doi: 10.1093/oxfordjournals.aje.a120179. [DOI] [PubMed] [Google Scholar]
- Meitert T., Pencu E., Ciudin L., Tonciu M. Vaccine strain Sh. flexneri T32-Istrati. Studies in animals and in volunteers. Antidysentery immunoprophylaxis and immunotherapy by live vaccine Vadizen (Sh. flexneri T32-Istrati). Arch Roum Pathol Exp Microbiol. 1984 Jul-Dec;43(3-4):251–278. [PubMed] [Google Scholar]
- Mel D. M., Arsić B. L., Nikolić B. D., Radovanić M. L. Studies on vaccination against bacillary dysentery. 4. Oral immunization with live monotypic and combined vaccines. Bull World Health Organ. 1968;39(3):375–380. [PMC free article] [PubMed] [Google Scholar]
- Mel D. M., Papo R. G., Terzin A. L., Vuksić L. Studies on vaccination against bacillary dysentery. 2. Safety tests and reactogenicity studies on a live dysentery vaccine intended for use in field trials. Bull World Health Organ. 1965;32(5):637–645. [PMC free article] [PubMed] [Google Scholar]
- Mel D., Gangarosa E. J., Radovanovic M. L., Arsic B. L., Litvinjenko S. Studies on vaccination against bacillary dysentery. 6. Protection of children by oral immunization with streptomycin-dependent Shigella strains. Bull World Health Organ. 1971;45(4):457–464. [PMC free article] [PubMed] [Google Scholar]
- Oaks E. V., Hale T. L., Formal S. B. Serum immune response to Shigella protein antigens in rhesus monkeys and humans infected with Shigella spp. Infect Immun. 1986 Jul;53(1):57–63. doi: 10.1128/iai.53.1.57-63.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SERENY B. A new method for the measurement of protective potency of dysentery vaccines. Acta Microbiol Acad Sci Hung. 1962;9:55–60. [PubMed] [Google Scholar]
- SERENY B. Acquired natural immunity following recovery from keratoconjunctivitis shigellosa. J Hyg Epidemiol Microbiol Immunol. 1959;3:292–305. [PubMed] [Google Scholar]
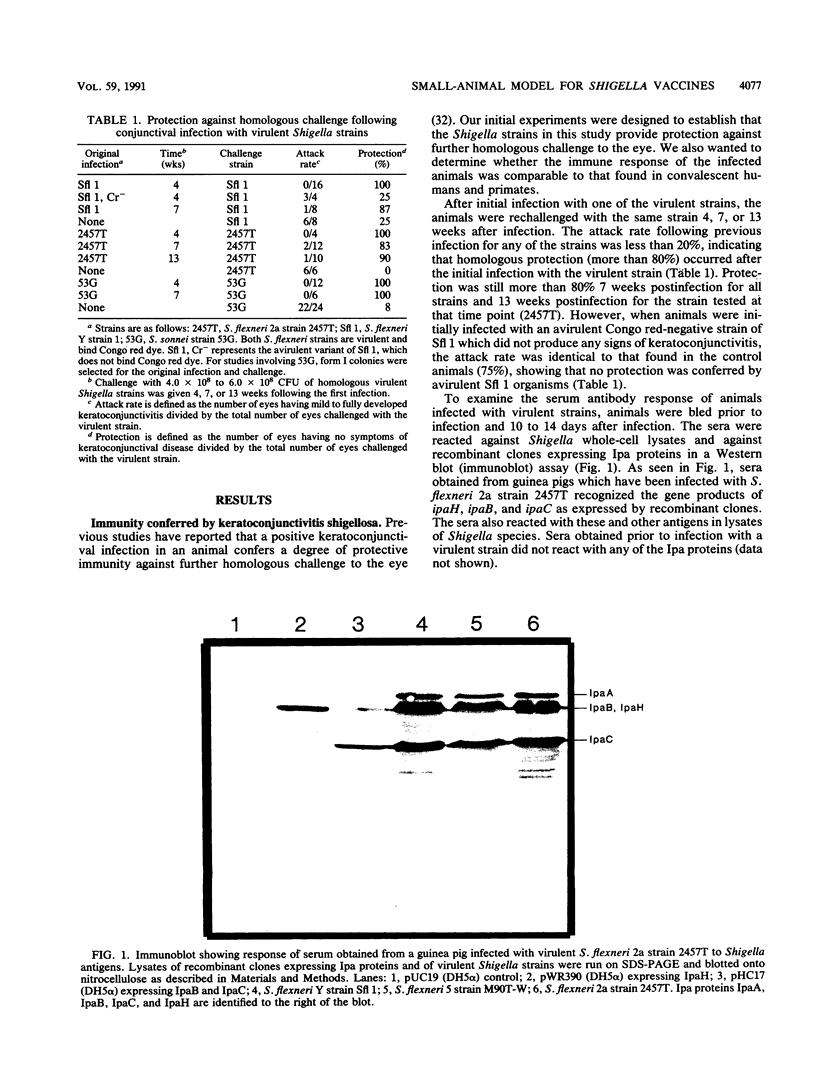
- SERENY B. Experimental keratoconjunctivitis shigellosa. Acta Microbiol Acad Sci Hung. 1957;4(4):367–376. [PubMed] [Google Scholar]
- Sansonetti P. J., Arondel J. Construction and evaluation of a double mutant of Shigella flexneri as a candidate for oral vaccination against shigellosis. Vaccine. 1989 Oct;7(5):443–450. doi: 10.1016/0264-410x(89)90160-6. [DOI] [PubMed] [Google Scholar]
- Van de Verg L., Herrington D. A., Murphy J. R., Wasserman S. S., Formal S. B., Levine M. M. Specific immunoglobulin A-secreting cells in peripheral blood of humans following oral immunization with a bivalent Salmonella typhi-Shigella sonnei vaccine or infection by pathogenic S. sonnei. Infect Immun. 1990 Jun;58(6):2002–2004. doi: 10.1128/iai.58.6.2002-2004.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatesan M. M., Buysse J. M., Kopecko D. J. Characterization of invasion plasmid antigen genes (ipaBCD) from Shigella flexneri. Proc Natl Acad Sci U S A. 1988 Dec;85(23):9317–9321. doi: 10.1073/pnas.85.23.9317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma N. K., Lindberg A. A. Construction of aromatic dependent Shigella flexneri 2a live vaccine candidate strains: deletion mutations in the aroA and the aroD genes. Vaccine. 1991 Jan;9(1):6–9. doi: 10.1016/0264-410x(91)90308-s. [DOI] [PubMed] [Google Scholar]