Abstract
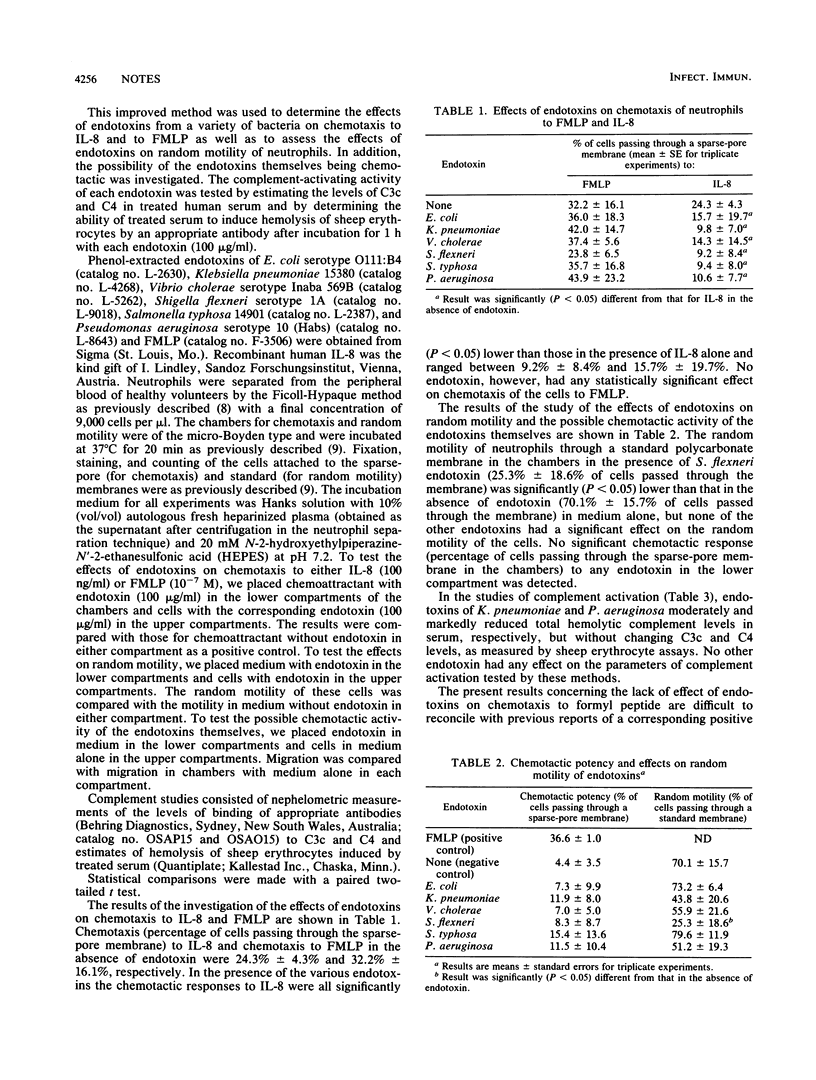
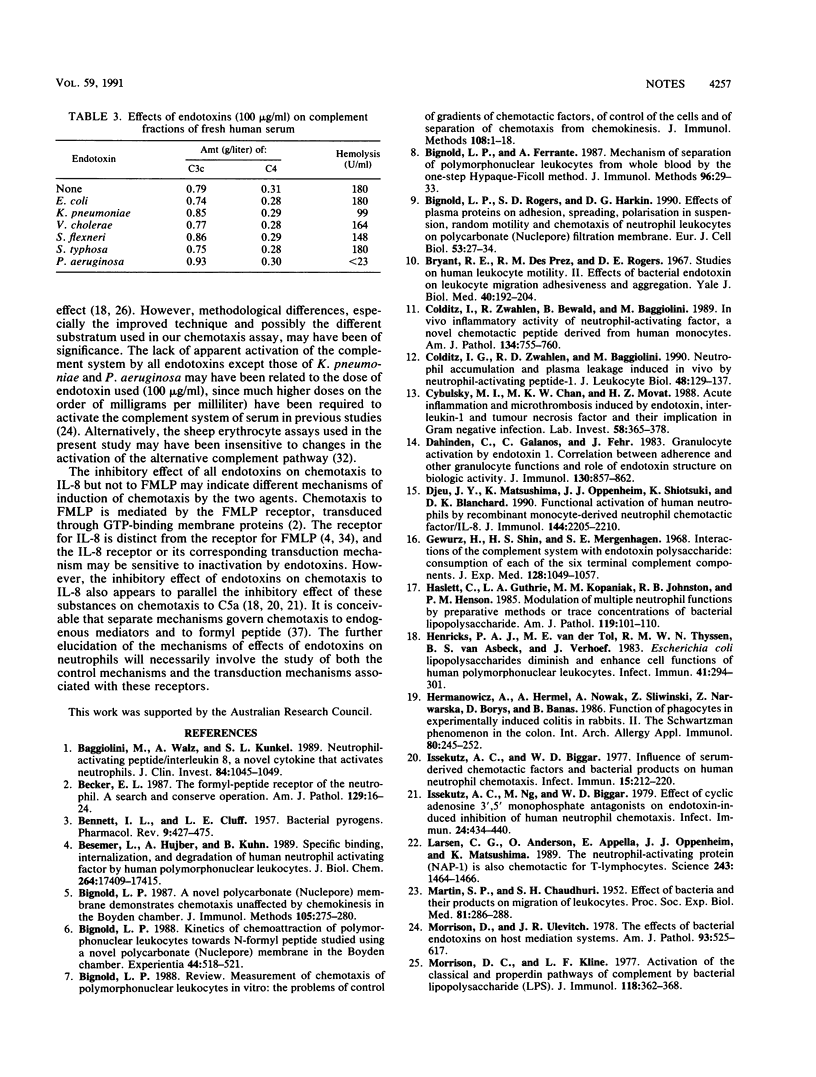
The effects of endotoxins from various bacteria (Escherichia coli, Klebsiella pneumoniae, Vibrio cholerae, Shigella flexneri, Salmonella typhosa, and Pseudomonas aeruginosa) on chemotaxis of neutrophil leukocytes to formyl peptide and interleukin-8 were tested in an improved chemotaxis assay involving a "sparse-pore" polycarbonate (Nuclepore) membrane in a Boyden-type chamber. The possible chemotactic activity of the endotoxins themselves were tested by the same technique. In addition, the effects of these substances on random motility of neutrophils were tested with a corresponding assay involving similar chambers fitted with membranes of standard pore density. Possible activation of the complement system of serum by each endotoxin was tested with sheep erythrocyte assays and the maximum endotoxin concentration (100 micrograms/ml) used in the chemotaxis and motility assays. All endotoxins inhibited chemotaxis of neutrophils to interleukin-8. No endotoxin affected chemotaxis to formyl peptide or was itself chemotactic for neutrophils. Endotoxin of S. flexneri inhibited random motility of neutrophils, while the others had no such effect. Endotoxins of K. pneumoniae and of P. aeruginosa produced moderate and marked inhibition, respectively, of total complement, as measured by hemolysis of sheep erythrocytes, without affecting the levels of C3c and C4 in these assays. Endotoxins of the other bacteria had no demonstrable effect in any of these assays of complement activation. These results suggest that chemotaxis to interleukin-8 may be mediated by cellular mechanisms different from those involved in chemotaxis to formyl peptide. Furthermore, the presence of these endotoxins could be significant for the suppression of neutrophil accumulation in inflammatory lesions mediated by interleukin-8.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BENNETT I. L., Jr, CLUFF L. E. Bacterial pyrogens. Pharmacol Rev. 1957 Dec;9(4):427–479. [PubMed] [Google Scholar]
- Baggiolini M., Walz A., Kunkel S. L. Neutrophil-activating peptide-1/interleukin 8, a novel cytokine that activates neutrophils. J Clin Invest. 1989 Oct;84(4):1045–1049. doi: 10.1172/JCI114265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker E. L. Rous-Whipple award lecture. The formylpeptide receptor of the neutrophil. A search and conserve operation. Am J Pathol. 1987 Oct;129(1):15–24. [PMC free article] [PubMed] [Google Scholar]
- Besemer J., Hujber A., Kuhn B. Specific binding, internalization, and degradation of human neutrophil activating factor by human polymorphonuclear leukocytes. J Biol Chem. 1989 Oct 15;264(29):17409–17415. [PubMed] [Google Scholar]
- Bignold L. P. A novel polycarbonate (Nuclepore) membrane demonstrates chemotaxis, unaffected by chemokinesis, of polymorphonuclear leukocytes in the Boyden chamber. J Immunol Methods. 1987 Dec 24;105(2):275–280. doi: 10.1016/0022-1759(87)90275-4. [DOI] [PubMed] [Google Scholar]
- Bignold L. P., Ferrante A. Mechanism of separation of polymorphonuclear leukocytes from whole blood by the one-step Hypaque-Ficoll method. J Immunol Methods. 1987 Jan 26;96(1):29–33. doi: 10.1016/0022-1759(87)90363-2. [DOI] [PubMed] [Google Scholar]
- Bignold L. P. Kinetics of chemo-attraction of polymorphonuclear leukocytes towards N-formyl peptide studied with a novel polycarbonate (Nucleopore) membrane in the Boyden chamber. Experientia. 1988 Jun 15;44(6):518–521. doi: 10.1007/BF01958935. [DOI] [PubMed] [Google Scholar]
- Bignold L. P. Measurement of chemotaxis of polymorphonuclear leukocytes in vitro. The problems of the control of gradients of chemotactic factors, of the control of the cells and of the separation of chemotaxis from chemokinesis. J Immunol Methods. 1988 Apr 6;108(1-2):1–18. doi: 10.1016/0022-1759(88)90396-1. [DOI] [PubMed] [Google Scholar]
- Bignold L. P., Rogers S. D., Harkin D. G. Effects of plasma proteins on the adhesion, spreading, polarization in suspension, random motility and chemotaxis of neutrophil leukocytes on polycarbonate (Nuclepore) filtration membrane. Eur J Cell Biol. 1990 Oct;53(1):27–34. [PubMed] [Google Scholar]
- Bryant R. E., Des Prez R. M., Rogers D. E. Studies on human leukocyte motility. II. Effects of bacterial endotoxin on leukocyte migration, adhesiveness, and aggregation. Yale J Biol Med. 1967 Dec;40(3):192–204. [PMC free article] [PubMed] [Google Scholar]
- Colditz I. G., Zwahlen R. D., Baggiolini M. Neutrophil accumulation and plasma leakage induced in vivo by neutrophil-activating peptide-1. J Leukoc Biol. 1990 Aug;48(2):129–137. doi: 10.1002/jlb.48.2.129. [DOI] [PubMed] [Google Scholar]
- Colditz I., Zwahlen R., Dewald B., Baggiolini M. In vivo inflammatory activity of neutrophil-activating factor, a novel chemotactic peptide derived from human monocytes. Am J Pathol. 1989 Apr;134(4):755–760. [PMC free article] [PubMed] [Google Scholar]
- Cybulsky M. I., Chan M. K., Movat H. Z. Acute inflammation and microthrombosis induced by endotoxin, interleukin-1, and tumor necrosis factor and their implication in gram-negative infection. Lab Invest. 1988 Apr;58(4):365–378. [PubMed] [Google Scholar]
- Dahinden C., Galanos C., Fehr J. Granulocyte activation by endotoxin. I. Correlation between adherence and other granulocyte functions, and role of endotoxin structure on biologic activity. J Immunol. 1983 Feb;130(2):857–862. [PubMed] [Google Scholar]
- Djeu J. Y., Matsushima K., Oppenheim J. J., Shiotsuki K., Blanchard D. K. Functional activation of human neutrophils by recombinant monocyte-derived neutrophil chemotactic factor/IL-8. J Immunol. 1990 Mar 15;144(6):2205–2210. [PubMed] [Google Scholar]
- Gewurz H., Shin H. S., Mergenhagen S. E. Interactions of the complement system with endotoxic lipopolysaccharide: consumption of each of the six terminal complement components. J Exp Med. 1968 Nov 1;128(5):1049–1057. doi: 10.1084/jem.128.5.1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haslett C., Guthrie L. A., Kopaniak M. M., Johnston R. B., Jr, Henson P. M. Modulation of multiple neutrophil functions by preparative methods or trace concentrations of bacterial lipopolysaccharide. Am J Pathol. 1985 Apr;119(1):101–110. [PMC free article] [PubMed] [Google Scholar]
- Henricks P. A., van der Tol M. E., Thyssen R. M., van Asbeck B. S., Verhoef J. Escherichia coli lipopolysaccharides diminish and enhance cell function of human polymorphonuclear leukocytes. Infect Immun. 1983 Jul;41(1):294–301. doi: 10.1128/iai.41.1.294-301.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermanowicz A., Hermel A., Nowak A., Sliwiński Z., Nawarska Z., Borys D., Banaś B. Function of phagocytes in experimentally induced colitis in rabbits. II. The Shwartzman phenomenon in the colon. Int Arch Allergy Appl Immunol. 1986;80(3):245–252. doi: 10.1159/000234060. [DOI] [PubMed] [Google Scholar]
- Issekutz A. C., Biggar W. D. Influence of serum-derived chemotactic factors and bacterial products on human neutrophil chemotaxis. Infect Immun. 1977 Jan;15(1):212–220. doi: 10.1128/iai.15.1.212-220.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Issekutz A. C., Ng M., Biggar W. D. Effect of cyclic adenosine 3',5'-monophosphate antagonists on endotoxin-induced inhibition of human neutrophil chemotaxis. Infect Immun. 1979 May;24(2):434–440. doi: 10.1128/iai.24.2.434-440.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen C. G., Anderson A. O., Appella E., Oppenheim J. J., Matsushima K. The neutrophil-activating protein (NAP-1) is also chemotactic for T lymphocytes. Science. 1989 Mar 17;243(4897):1464–1466. doi: 10.1126/science.2648569. [DOI] [PubMed] [Google Scholar]
- MARTIN S. P., CHAUDHURI S. N. Effect of bacteria and their products on migration of leukocytes. Proc Soc Exp Biol Med. 1952 Oct;81(1):286–288. doi: 10.3181/00379727-81-19851. [DOI] [PubMed] [Google Scholar]
- Morrison D. C., Kline L. F. Activation of the classical and properdin pathways of complement by bacterial lipopolysaccharides (LPS). J Immunol. 1977 Jan;118(1):362–368. [PubMed] [Google Scholar]
- Morrison D. C., Ulevitch R. J. The effects of bacterial endotoxins on host mediation systems. A review. Am J Pathol. 1978 Nov;93(2):526–618. [PMC free article] [PubMed] [Google Scholar]
- Nitzan D. W., Pruzanski W., Saito S., Ranadive N. Modulation of locomotor activity of polymorphonuclear cells by cationic substances and cationic lysosomal fractions from human neutrophils. Inflammation. 1985 Dec;9(4):375–387. doi: 10.1007/BF00916337. [DOI] [PubMed] [Google Scholar]
- Peveri P., Walz A., Dewald B., Baggiolini M. A novel neutrophil-activating factor produced by human mononuclear phagocytes. J Exp Med. 1988 May 1;167(5):1547–1559. doi: 10.1084/jem.167.5.1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raetz C. R. Biochemistry of endotoxins. Annu Rev Biochem. 1990;59:129–170. doi: 10.1146/annurev.bi.59.070190.001021. [DOI] [PubMed] [Google Scholar]
- Rampart M., Van Damme J., Zonnekeyn L., Herman A. G. Granulocyte chemotactic protein/interleukin-8 induces plasma leakage and neutrophil accumulation in rabbit skin. Am J Pathol. 1989 Jul;135(1):21–25. [PMC free article] [PubMed] [Google Scholar]
- Rosenbaum J. T., Hartiala K. T., Webster R. O., Howes E. L., Jr, Goldstein I. M. Antiinflammatory effects of endotoxin. Inhibition of rabbit polymorphonuclear leukocyte responses to complement (C5)-derived peptides in vivo and in vitro. Am J Pathol. 1983 Dec;113(3):291–299. [PMC free article] [PubMed] [Google Scholar]
- Samanta A. K., Oppenheim J. J., Matsushima K. Identification and characterization of specific receptors for monocyte-derived neutrophil chemotactic factor (MDNCF) on human neutrophils. J Exp Med. 1989 Mar 1;169(3):1185–1189. doi: 10.1084/jem.169.3.1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samanta A. K., Oppenheim J. J., Matsushima K. Interleukin 8 (monocyte-derived neutrophil chemotactic factor) dynamically regulates its own receptor expression on human neutrophils. J Biol Chem. 1990 Jan 5;265(1):183–189. [PubMed] [Google Scholar]
- Schröder J. M., Mrowietz U., Morita E., Christophers E. Purification and partial biochemical characterization of a human monocyte-derived, neutrophil-activating peptide that lacks interleukin 1 activity. J Immunol. 1987 Nov 15;139(10):3474–3483. [PubMed] [Google Scholar]
- Sveen K. The importance of C5 and the role of the alternative complement pathway in leukocyte chemotaxis induced in vivo and in vitro by Bacteroides fragilis lipopolysaccharide. Acta Pathol Microbiol Scand B. 1978 Apr;86(2):93–100. doi: 10.1111/j.1699-0463.1978.tb00015.x. [DOI] [PubMed] [Google Scholar]
- Ternowitz T., Herlin T., Fogh K. Human monocyte and polymorphonuclear leukocyte chemotactic and chemokinetic responses to leukotriene B4 and FMLP. Acta Pathol Microbiol Immunol Scand C. 1987 Apr;95(2):47–54. doi: 10.1111/j.1699-0463.1987.tb00008.x. [DOI] [PubMed] [Google Scholar]
- Thelen M., Peveri P., Kernen P., von Tscharner V., Walz A., Baggiolini M. Mechanism of neutrophil activation by NAF, a novel monocyte-derived peptide agonist. FASEB J. 1988 Aug;2(11):2702–2706. [PubMed] [Google Scholar]
- Van Damme J., Van Beeumen J., Conings R., Decock B., Billiau A. Purification of granulocyte chemotactic peptide/interleukin-8 reveals N-terminal sequence heterogeneity similar to that of beta-thromboglobulin. Eur J Biochem. 1989 May 1;181(2):337–344. doi: 10.1111/j.1432-1033.1989.tb14729.x. [DOI] [PubMed] [Google Scholar]
- Van Damme J., Van Beeumen J., Opdenakker G., Billiau A. A novel, NH2-terminal sequence-characterized human monokine possessing neutrophil chemotactic, skin-reactive, and granulocytosis-promoting activity. J Exp Med. 1988 Apr 1;167(4):1364–1376. doi: 10.1084/jem.167.4.1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verghese M. W., Snyderman R. Differential anti-inflammatory effects of LPS in susceptible and resistant mouse strains. J Immunol. 1981 Jul;127(1):288–293. [PubMed] [Google Scholar]
- Walz A., Peveri P., Aschauer H., Baggiolini M. Purification and amino acid sequencing of NAF, a novel neutrophil-activating factor produced by monocytes. Biochem Biophys Res Commun. 1987 Dec 16;149(2):755–761. doi: 10.1016/0006-291x(87)90432-3. [DOI] [PubMed] [Google Scholar]
- Westwick J., Li S. W., Camp R. D. Novel neutrophil-stimulating peptides. Immunol Today. 1989 May;10(5):146–147. doi: 10.1016/0167-5699(89)90164-3. [DOI] [PubMed] [Google Scholar]
- Wilson M. E. Effects of bacterial endotoxins on neutrophil function. Rev Infect Dis. 1985 May-Jun;7(3):404–418. doi: 10.1093/clinids/7.3.404. [DOI] [PubMed] [Google Scholar]
- Yoshimura T., Matsushima K., Tanaka S., Robinson E. A., Appella E., Oppenheim J. J., Leonard E. J. Purification of a human monocyte-derived neutrophil chemotactic factor that has peptide sequence similarity to other host defense cytokines. Proc Natl Acad Sci U S A. 1987 Dec;84(24):9233–9237. doi: 10.1073/pnas.84.24.9233. [DOI] [PMC free article] [PubMed] [Google Scholar]


