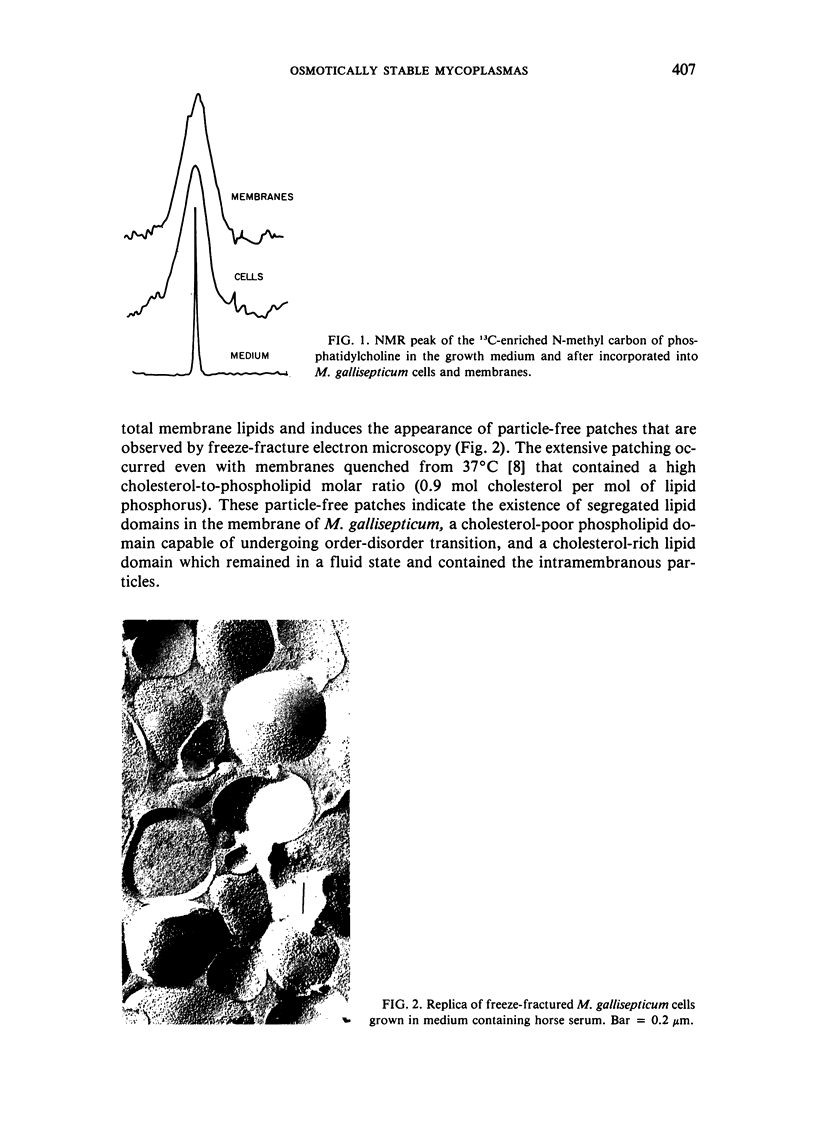
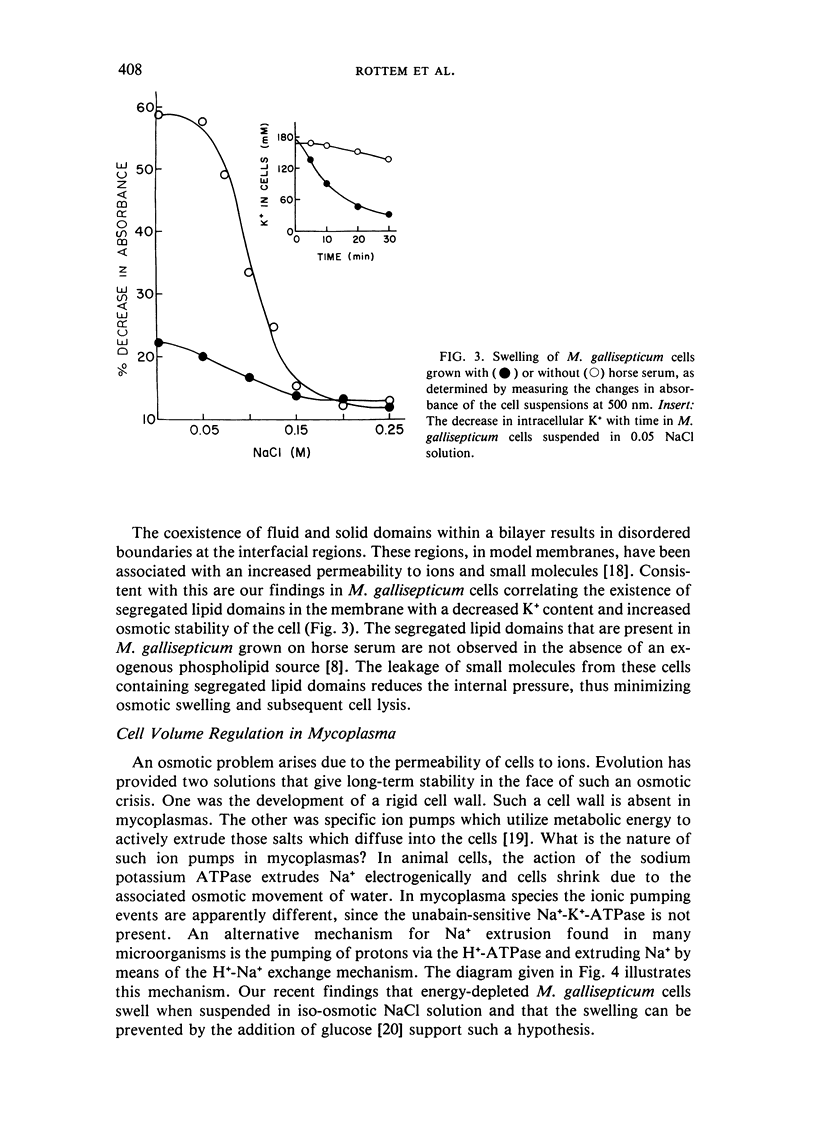
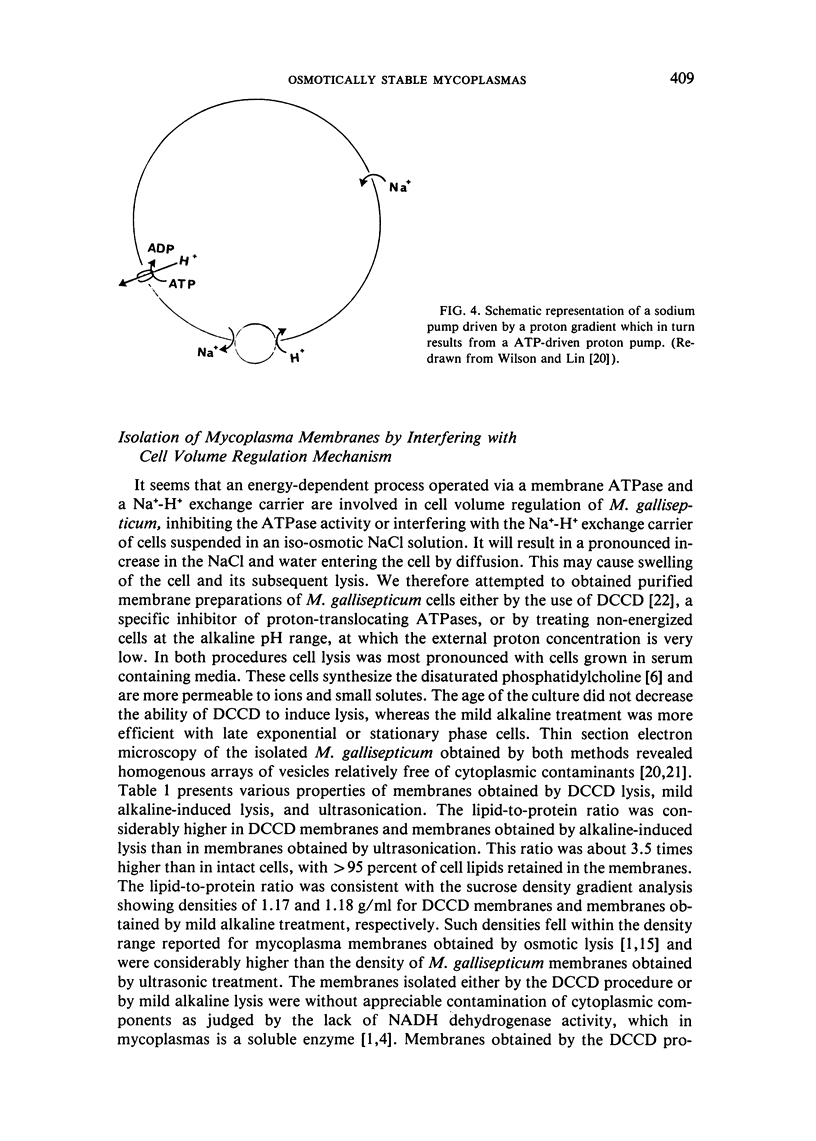
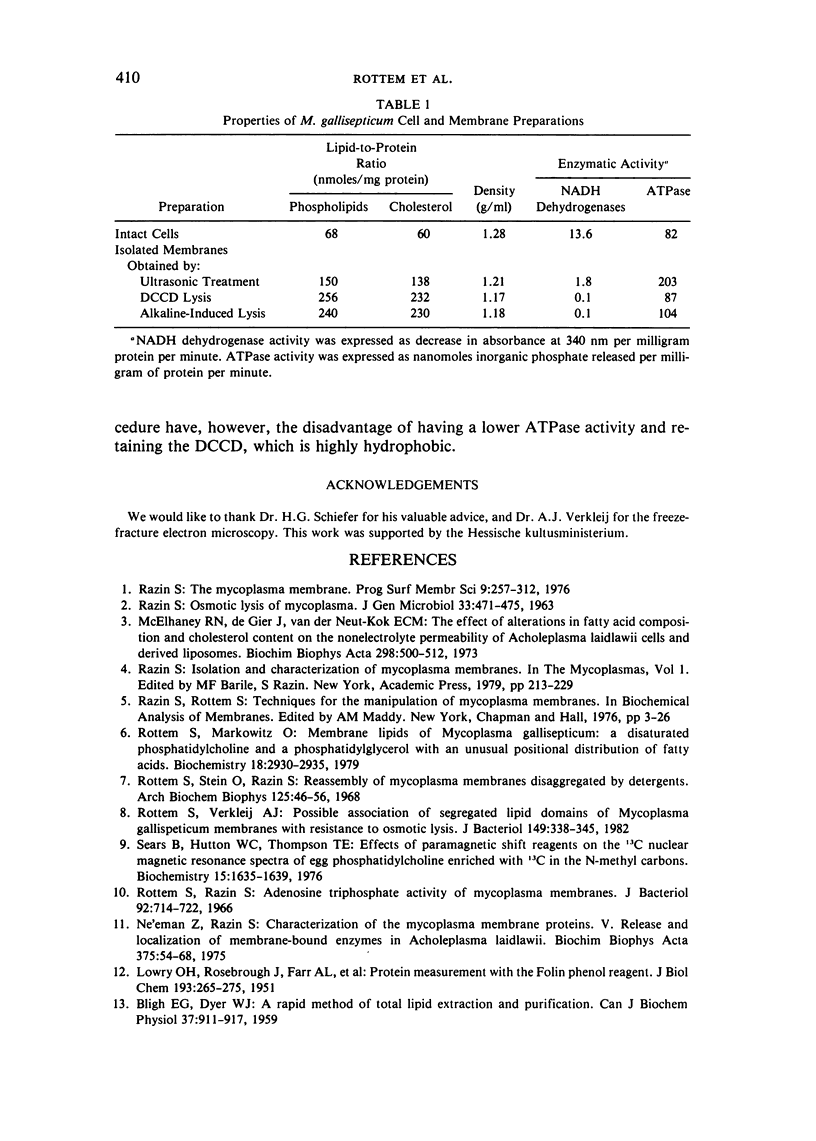
Abstract
The osmotic stability of M. gallisepticum was found to be a consequence of the synthesis of disaturated phosphatidylcholine incorporated into the cell membrane. The disaturated lipid induces the formation of segregated lipid domains, thus providing the sites for increased permeation of ions. Such permeation reduces the internal pressure so as to minimize cell swelling and subsequent lysis in a hypotonic medium. Purified membranes of M. gallisepticum can be prepared from cells suspended in an iso-osmotic NaCl solution containing either dicyclohexylcarbodiimide (DCCD), which blocks ATPase activity, or a mild alkaline buffer. Both conditions seem to interfere with cell volume regulation. These procedures can be used also to isolate membranes of other osmotically stable mycoplasmas.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Marsh D., Watts A., Knowles P. F. Evidence for phase boundary lipid. Permeability of Tempo-choline into dimyristoylphosphatidylcholine vesicles at the phase transition. Biochemistry. 1976 Aug 10;15(16):3570–3578. doi: 10.1021/bi00661a027. [DOI] [PubMed] [Google Scholar]
- Mcelhaney R. N., de Gier J., van der Neut-Kok E. C. The effect of alterations in fatty acid composition and cholesterol content on the nonelectrolyte permeability of Acholeplasma laidlawii B cells and derived liposomes. Biochim Biophys Acta. 1973 Mar 16;298(2):500–512. doi: 10.1016/0005-2736(73)90376-3. [DOI] [PubMed] [Google Scholar]
- Ne'eman Z., Razin S. Characterization of the mycoplasma membrane proteins. V. Release and localization of membrane-bound enzymes in Acholeplasma laidlawii. Biochim Biophys Acta. 1975 Jan 14;375(1):54–68. doi: 10.1016/0005-2736(75)90072-3. [DOI] [PubMed] [Google Scholar]
- RAZIN S. OSMOTIC LYSIS OF MYCOPLASMA. J Gen Microbiol. 1963 Dec;33:471–475. doi: 10.1099/00221287-33-3-471. [DOI] [PubMed] [Google Scholar]
- Razin S., Kutner S., Efrati H., Rottem S. Phospholipid and cholesterol uptake by Mycoplasma cells and membranes. Biochim Biophys Acta. 1980 Jun 6;598(3):628–640. doi: 10.1016/0005-2736(80)90042-5. [DOI] [PubMed] [Google Scholar]
- Rottem S., Linker C., Wilson T. H. Proton motive force across the membrane of Mycoplasma gallisepticum and its possible role in cell volume regulation. J Bacteriol. 1981 Mar;145(3):1299–1304. doi: 10.1128/jb.145.3.1299-1304.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rottem S., Markowitz O. Membrane lipids of Mycoplasma gallisepticum: a disaturated phosphatidylcholine and a phosphatidylglycerol with an unusual positional distribution of fatty acids. Biochemistry. 1979 Jul 10;18(14):2930–2935. doi: 10.1021/bi00581a002. [DOI] [PubMed] [Google Scholar]
- Rottem S. Membrane lipids of mycoplasmas. Biochim Biophys Acta. 1980 May 27;604(1):65–90. doi: 10.1016/0005-2736(80)90585-4. [DOI] [PubMed] [Google Scholar]
- Rottem S., Razin S. Adenosine triphosphatase activity of mycoplasma membranes. J Bacteriol. 1966 Sep;92(3):714–722. doi: 10.1128/jb.92.3.714-722.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rottem S., Stein O., Razin S. Reassembly of Mycoplasma membranes disaggregated by detergents. Arch Biochem Biophys. 1968 Apr;125(1):46–56. doi: 10.1016/0003-9861(68)90637-1. [DOI] [PubMed] [Google Scholar]
- Rottem S., Verkleij A. J. Possible association of segregated lipid domains of Mycoplasma gallisepticum membranes with cell resistance to osmotic lysis. J Bacteriol. 1982 Jan;149(1):338–345. doi: 10.1128/jb.149.1.338-345.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rottem S., Verkleij A. J. Possible association of segregated lipid domains of Mycoplasma gallisepticum membranes with cell resistance to osmotic lysis. J Bacteriol. 1982 Jan;149(1):338–345. doi: 10.1128/jb.149.1.338-345.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudel L. L., Morris M. D. Determination of cholesterol using o-phthalaldehyde. J Lipid Res. 1973 May;14(3):364–366. [PubMed] [Google Scholar]
- Sears B., Hutton W. C., Thompson T. E. Effects of paramagnetic shift reagents on the 13C nuclear magnetic resonance spectra of egg phosphatidylcholine enriched with 13C in the N-methyl carbons. Biochemistry. 1976 Apr 20;15(8):1635–1639. doi: 10.1021/bi00653a007. [DOI] [PubMed] [Google Scholar]
- Wilson T. H., Lin E. C. Evolution of membrane bioenergetics. J Supramol Struct. 1980;13(4):421–446. doi: 10.1002/jss.400130403. [DOI] [PubMed] [Google Scholar]



