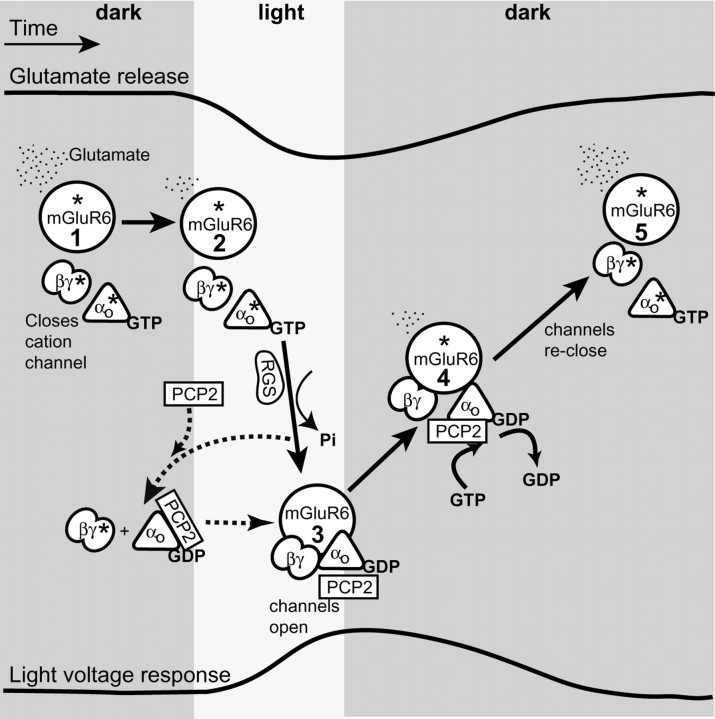
Figure 8.
Schematic representation of the G-protein cycle and the possible function of PCP2 in modulating the light response in ON bipolar cells. The top trace represents glutamate release over time, and the bottom trace represents voltage response of a rod bipolar cell. The solid arrows denote transition between known steps (which are numbered within mGluR6 circles) in the cascade; the dotted lines denote possible interactions. The asterisk (*) denotes active state of a protein. Steps 1 and 5, In darkness, continuous release of glutamate from photoreceptors activates the mGluR6 receptors located at the dendritic tips of the ON bipolar cells. Activation of mGluR6 leads to an exchange of GDP with GTP on Gαo1 and dissociation of Gβγ from GαGTP. As a result, GαGTP and Gβγ are in their active forms, and either of them can, in principle, lead to closure of an unidentified cation channel and membrane hyperpolarization. Step 2, When light intensity increases, glutamate release decreases, and the intrinsic GAP activity of Gα hydrolyzes GαGTP. This hydrolysis in bipolar cells is likely facilitated by an RGS protein. Step 3, Consequently, [GαGDP] increases and rebinds Gβγ forming the inactive heterotrimer. Thus, channels are allowed to open and the membrane depolarizes. Step 4, When the light is turned off, glutamate release increases again, Gαo1 is reactivated by a GDP to GTP exchange on Gαo1, the channels close, and the light response is terminated. The hyperpolarization by PCP2 (in steps 1 and 5) (i.e., the increase in the fraction of channels closed by glutamate in presence of PCP2) suggests that a larger fraction of the G-protein heterotrimer is active. This may result either by GEF mechanism (activating both Gαo and Gβγ) or a GDI (activating Gβγ by binding Gαo GDP). The acceleration of time to peak by PCP2 (transition from steps 2 and 3) may result either from an actual acceleration of the G-protein deactivation or from sharpening the peak. In the first case, acceleration of deactivation may result if PCP2 acts as GDI: by capturing Gαo GDP, it may reduce Gαo available for reactivation by residual glutamate. In the second case, the peak may be sharpened by repolarizing as soon as glutamate concentration increases and the G-protein starts to reactivate (transition from steps 3 and 4). This is easiest to explain if PCP2 is a GEF. PCP2 seems to affect the main component of the response decay (transition from steps 4 and 5) not by directly acting on the excitatory current, but via the inhibitory network, which feeds back onto the rod bipolar cell and accelerates the termination of the response.