Table 1.
Reaction Conditions: Carboxylate, Solvent, Temperature
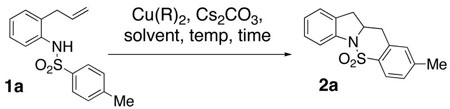 | ||||
|---|---|---|---|---|
| entry | R | solvent | temp, time | yield (%)d |
| 1 | Oac | DMF | 120 °C,b 24 h | 69 |
| 2 | Oac | CH3CN | 120 °C,b 24 h | 73 |
| 3 | Oac | i-PrOH | 120 °C,b 24 h | 16e |
| 4 | OAc | t-amyl-OH | 120 °C,b 24 h | 33 |
| 5 | OAc | EtOAc | 120 °C,b 24 h | 24e |
| 6 | OAc | toluene | 120 °C,b 24 h | 51e |
| 7 | OAc | DMA | 120 °C,b 24 h | 37 |
| 8 | OPiv | DMF | 120 °C,b 24 h | 69 |
| 9 | EH | DMF | 120 °C,b 24 h | 64 |
| 10 | ND | DMF | 120 °C,b 24 h | 69 |
| 11 | ND | toluene | 120 °C,b 24 h | 71 |
| 12 | ND | DMF | 120 °C,b 0.5 h | 29e |
| 13 | ND | DMF | 120 °C,c 0.5 h | 27e |
| 14 | ND | DMF | 160 °C,b 0.5 h | 63 |
| 15 | ND | DMF | 160 °C,c 0.5 h | 64 |
All reactions were run with 3 equiv copper(II) carboxylate, 1 equiv Cs2CO3 at 0.1 M 1a concentration.
Oil bath heating, pressure tube.
Microwave heating.
Amount isolated after flash chromatography on SiO2.
Remainder of the material is starting 1a. In entry 3 another cyclization product (net aminoetherification, incorporation of i-PrOH in the product) was also formed in 21% yield (see supplementary material). Ac = COCH3, Piv = COC(CH3)3, neodecanoate (ND) = OCO(CH2)5C(CH3)3, ethylhexanoate (EH) = OCOCH(C2H5)C4H9
