Table 4.
Diastereoseletive Formation of 2,5-Cis-Pyrrolidinesa
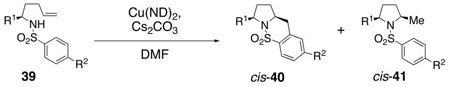 | ||||
|---|---|---|---|---|
| entry | substrate | method, temp, time | yield (%)c40 | yield (%)c41 |
| 1 | 39a, R1 = i-Pr, R2 = Me | oil bath, 190 °C, 72 h | 49 | 25 |
| 2 | 39a | 210 °C (μW), 3 h | 51 | 28 |
| 3 | 39a | oil bath, 200°C, 3 h | 40 | 19 |
| 4 | 39b, R1 = i-Pr, R2 = OMe | oil bath,190 °C, 72 h | 49 | 23 |
| 5 | 39b | 210 °C (μW), 3 h | 51 | 23 |
| 6 | 39b | oil bath, 190 °C, 72 hb | 33 | 24 |
| 7 | 39c. R1 = t-Bu, R2 = Me | oil bath, 170 °C, 72 h | 34 | 15 |
| 8 | 39c | 210 °C (μW), 3 h | 31 | 17 |
| 9 | 39d, R1 = Me, R2 = Me | oil bath, 200 °C, 72 h | 48 | 15 |
| 10 | 39d | 210 °C (μW), 3 h | 48 | 15 |
| 11 | 39e, R1 = i-PrCH2, R2 = Me | oil bath, 200 °C, 72 h | 49 | 20 |
| 12 | 39e | 210 °C (μW), 3 h | 49 | 19 |
| 13 | 39f, R1 = Bn, R2 = Me | oil bath, 200 °C, 72 h | 50 | 22 |
| 14 | 39f | 210 °C (μW), 3 h | 47 | 21 |
Sulfonamides 39 were dissolved in DMF (0.1 M) and treated with Cs2CO3 (1 equiv) and Cu(ND)2 (3 equiv) and were heated in the indicated manner at the indicated temperature and time.
Reaction run with NaH as base instead of Cs2CO3: Sulfamide 39b in DMF was treated with NaH (1.2 equiv) at 23 °C for 0.5 h, then Cu(ND)2 (1.2 equiv) in DMF was added, stirred 0.5 h, reaction was then heated at 190 °C for 72 h.
Yields refer to isolated products, diastereomeric ratios (>20 :1) were determined by analysis of the crude 1H NMR and by isolated yields. ND = neodecanoate.
